Abstract
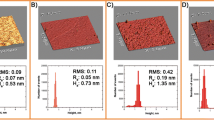
The glycosylphosphatidyl inositol (GPI)-anchored proteins are localized on the outer of the plasma membrane and clustered in membrane microdomain known as lipid rafts. Among them, mammalian alkaline phosphatase (AP) is an enzyme widely distributed. So, it has important biological significance to study the combination of AP with lipid monolayer. In our work, the interaction between AP and sphingomyelin has been studied at the air-buffer interface as a biomimetic membrane system by the Langmuir film technique and atomic force microscopy. The surface pressure-area isotherm for the mixed alkaline phosphatase/sphingomyelin monolayer shown the presence of a transition from a liquid-expanded phase to the liquid-expanded/liquid-condensed coexist phase. And the surface compressional modulus suggested the mixed alkaline phosphatase/sphingomyelin monolayer has larger compressibility compared with the pure sphingomyelin monolayer. Besides, according to the micrographs, we inferred when combined with lipid monolayer at the air-buffer interface, the AP molecules formed polymer not multilayer or micelle. And, according to the limiting molecules area of AP, we inferred that 12 AP molecules formed a hexagon polymer unit.
Similar content being viewed by others
Referemces
Alexandra M, Christine K, Megan SV, Judith VH, Martin S. Selective and programmed cleavage of GPI-anchored proteins from the surface membrane by phospholipase C. Biochimica et Biophysica Acta, 2012, 1818: 117–124
Ronzon F, Desbat B, Chauvet JP, Roux B. Behavior of a GPI-anchored protein in phospholipid monolayers at the air-water interface. Biochimica et Biophysica Acta, 2002, 1560: 1–13
Calvez P, Bussieres S, Demers E, Salesse C. Parameters modulating the maximum insertion pressure of proteins and peptides in lipid monolayers. Biochimie, 2009, 91: 718–733
Yasuko T, Yuko N, Hironobu H, Yoshiaki N. Studies directed toward the synthesis of protein-bound GPI anchor. Tetrahedron, 2003, 59: 4059–4067
Morihisa F, Taroh K. GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochimica et Biophysica Acta, 2012, 1821:1050–1058
Christoph M, Brian S, Walter H, John A. Danger field rafts anchors and viruses: a role for glycosylphosphatidyl inositol anchored proteins in the modification of enveloped viruses and viral vectors. Virology, 2008, 382: 125–131
Calvez P, Bussieres S, Demers E, Salesse C. Parameters modulating the maximum insertion pressure of proteins and peptides in lipid monolayers. Biochimie, 2009, 91: 718–733
Fernando LC, Katia RPD, Prislaine PM, João MP, Pietro C. Construction of an alkaline phosphatase-liposome system: a tool for biomineralization study. Int J Biochem Cell B, 2002, 34: 1091–1101
Bolean M, Simão AMS, Favarin BZ, Millán JL, Ciancaglini P. Thermodynamic properties and characterization of proteoliposomes rich in microdomains carrying alkaline phosphatase. Biophys Chem, 2011, 158: 111–118
Bolean M, Simão AMS, Favarin BZ, Millán JL, Ciancaglini P. The effect of cholesterol on the reconstitution of alkaline phosphatase into liposomes. Biophys Chem, 2010, 152: 74–79
Daniela FI, Joao MP, Pietro C. Erythrocyte ghost cell-alkaline phosphatase: construction and characterization of a vesicular system for use in biomineral ization studies. Biochimica et Biophysica Acta, 2002, 1567: 183–192
Caseli L, Zaniquelli MED, Furriel RPM, Leone FA. Enzymatic activity of alkaline phosphatase adsorbed on dimyristoylphosphatidic acid Langmuir-Blodgett films. Colloid Surface B, 2002, 25: 119–128
Caseli L, Maria DZ, Furriel RPM, Leone FA, Zaniquelli MED, Orbulescu J, Leblanc RM. Rat osseous plate alkaline phosphatase as Langmuir monolayer: an infrared study at the air-water interface. J Colloid Interf Sci, 2008, 320: 476–482
Agnes PG, Stephanie G, Loic JB. Enzyme association with lipidic Langmuir-Blodgett films: interests and applications in nanobioscience. Adv Colloid Interfac Sci, 2005, 116: 205–225
Luciano C, Douglas CM, Rosa PMF, Francisco AL, Maria EDZ. Influence of the glycosylphosphatidylinositol anchor in the morphology and roughness of Langmuir-Blodgett films of phospholipids containing alkaline phosphatases. Thin Solid Films, 2007, 515: 4801–4807
Frederic R, Bernard D, Jean-Paul C, Bernard R. Penetration of a GPI-anchored protein into phospholipid monolayers spread at the air/water interface. Colloid Surface B, 2002, 23: 365–373
Achraf K, Francoise B. GPI-alkaline phosphatase insertion into phosphatidylcholine monolayers: phase behavior and morphology changes. Biochem Bioph Res Co, 2005, 333: 1315–1321
Luciano C, Douglas CM, Rosa PMF, Francisco AL, Maria EDZ. Incorporation conditions guiding the aggregation of a glycosylphosphatidylinositol (GPI)-anchored protein in Langmuir monolayers. Colloid Surface B, 2005, 46: 248–254
Zheludeva S, Novikova N, Stepina N, Yurieva E, Konovalov O. Molecular organization in protein-lipid film on the water surface studied by x-ray standing wave measurements under total external reflection. Spectrochim Acta B, 2008, 63: 1399–1403
Luciano C, Frank NC, Thatyane MN, Maria EDZ, Valtencir Z, Osvaldo Jr NO. Using phospholipid Langmuir and Langmuir-Blodgett films as matrix for urease immobilization. J Colloid Interf Sci, 2008, 319: 100–108
Peng JB, Barnes GT, Gentle IR. The structures of Langmuir-Blodgett films of fatty acids and their salts. Adv Colloid Interface Sci, 2001, 91: 163–219
Gerhard S, Jiayun Zh. Chain length dependence of lipid partitioning between the air/water interface and its subphase. Chem Phys Lipids, 2001, 110: 35–45
Hao CC, Sun RG, Zhang J, Chang YG, Niu CL. Behavior of sulfatide/cholesterol mixed monolayers at the air/water interface. Colloid Surface B, 2009, 69: 201–206
Zakanda FN, Laurent P, Paquot M, Lelo GM, Deleu M. Alkylbetainate chlorides: synthesis and behavior of monolayers at the-air-water interface. Thin Solid Films, 2011, 520: 344–350
Amiya Kumar P, Vasilev K, Orgeig S, Clive AP. Thermodynamic and structural studies of mixed monolayers: mutual mixing of DPPC and DPPG with DTAP at the air-water interface. Mater Sci Eng C, 2010, 30: 542–548
Karen S, Juha-Pekka M, Paavo KJK. Interfacial behavior of cholesterol, ergosterol, and lanosterol in mixtures with DPPC and DMPC. Biophys J, 2008, 95: 2340–2355
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, J., Sun, R. & Hao, C. Mixed alkaline phosphatase/sphingomyelin monolayer at the air-buffer interface: phase behavior and morphology. Sci. China Chem. 57, 1538–1543 (2014). https://doi.org/10.1007/s11426-014-5124-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5124-9