Abstract
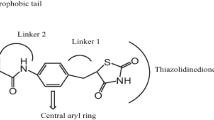
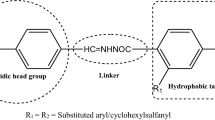
The synthesis of two series of β-amino ketones containing a p-aminobenzoic acid moiety (TM-1 and TM-2) using a modified protocol of the Mannich reaction is reported. The molecular structures of a total of tweenty three new target compounds were characterized by 1H NMR, 13C NMR, ESI-MS and HR-MS. Subsequently, their antidiabetic activities were screened in vitro. The α-glucodase inhibition (α-GI) activity of compound 1e reached a remarkable level of 66.50%. The peroxisome proliferator-activated receptor (PPAR) relative activation activities of six compounds are above 80%, and in particular 2i displays an unprecedentedly high PPAR of 130.91%. The structure-activity relationships of the compounds were established. 2i is also subject to further in-depth investigation.
Similar content being viewed by others
References
Ottanà R, Maccari R, Giglio M, Corso AD, Cappiello M, Mura U, Cosconati S, Marinelli L, Novellino E, Sartini S, Motta CL, Settimo FD. Identification of 5-arylidene-4-thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. Eur J Med Chem, 2011, 46: 2797–2806
Liu KC, Sakya SM, O’Donnell CJ, Flick AC, Ding HX. Synthetic approaches to the 2010 new drugs. Bioorg Med Chem, 2012, 20: 1155–1174
http://en.wikipedia.org/wiki/Linagliptin/GEN, News Highlights, 2011-05-03
Jorgensen MR, Bhurruth-Alcor Y, Røst T, Bohov P, Müller M, Guisado C, Kostarelos K, Dyrøy E, Berge RK, Miller AD, Skorve J. Synthesis and analysis of novel glycerolipids for the treatment of metabolic syndrome. J Med Chem, 2009, 52: 1172–1179
Shinkai H, Nishikawa M, Sato Y, Toi K, Kumashiro I, Seto Y, Fukuma M, Dan K, Toyoshima S. N-(Cyclohexyl-carbonyl)-d-phenylalanines and related compounds: A new class of oral hypoglycemic agents. J Med Chem, 1989, 32: 1436–1441
Grell W, Hurnaus R, Griss G, Sauter R, Rupprecht E, Mark M, Luger P, Nar H, Wittneben H, Müller P. Repaglinide and related hypoglycemic benzoic acid derivatives. J Med Chem, 1998, 41: 5219–5246
Henke BR, Blanchard SG, Brackeen MF, Brown KK, Cobb JE, Collins JL, Harrington WW, Hashim MA, Hull-Ryde EA, Kaldor I, Kliewer SA, Lake DH, Leesnitzer LM, Lehmann JM, Lenhard JM, Orband-Miller LA, Miller JF, Mook RA, Noble SA, Oliver W, Parks DJ, Plunket KD, Szewczyk JR, Willson TM. N-(2-benzoylphenyl)-l-tyrosine PPAR γ agonists. 1. Discovery of a novel series of potent antihyperglycemic and anti-hyperlipidemic agents. J Med Chem, 1998, 41: 5020–5036
Lohray BB, Bhushan V, Rao BP, Madhavan GR, Murali N, Rao KN, Reddy AK, Rajesh BM, Reddy PG, Chakrabarti R, Vikramadithyan RK, Rajagopalan R, Mamidi RN, Jajoo HK, Subramaniam S. Novel euglycemic and hypolipidemic agents. 1. J Med Chem, 1998, 41: 1619–1630
Reginato MJ, Bailey ST, Krakow SL, Minami KC, Ishii S, Tanaka H, Lazar MA. A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor γ-activating prop-erties. J Biol Chem, 1998, 273: 32679–32684
Klopfenstein SR, Evdokimov AG, Colson A, Fairweather NT, Neuman JJ, Maier MB, Gray JL, Gerwe GS, Stake GE, Howard BW, Farmer JA, Pokross ME, Downs TR, Kasibhatla B, Peters K. 1, 2, 3, 4-Tetrahydro-isoquinolinyl sulfamic acids as phosphatase PTP1B inhibitors. Bioorg Med Chem Lett, 2006, 16: 1574–1578
Wan ZK, Lee J, Xu WX, Erbe DV, Joseph-McCarthy D, Follows BC, Zhang YL. Monocyclic thiophenes as protein tyrosine phosphatase 1B inhibitors: Capturing interactions with Asp48. Bioorg Med Chem Lett, 2006, 16: 4941–4945
Li BN, Cai SZ, Du DM, Xu JX. Synthesis of phosphinopeptides via the Mannich ligation. Org Lett, 2007, 9: 2257–2260
Lautens M, Tayama E, Nguyen D. Direct vinylogous Mannich-type reactions via ring opening and rearrangement of vinyloxiranes. Org Lett, 2004, 6: 345–347
He M, Pan ZX, Song B, Zhang YP, Jin LH, Hu DY, Yang S, Song BA. Enantioselective synthesis of β-amino esters bearing a quinazoline moiety via a Mannich-type reaction catalyzed by a cinchona alkaloid derivative. Sci China Chem, 2012, 10. 1007/s11426-012-4822-4
Qiao CH, Xu XJ. The Beckmann rearrangement of 2-arylaminobenzyl (or methyl) cyclohexanone oxime. J Beijing Normal Univ (Nat Sci), 1998, 34: 89–92
He XW, Shang YJ, Hu JS, Ju K, Jiang W, Wang SF. Syntheses of N-sulfonyl-N,N-disubstituted amidines via a three-component free-radical coupling reaction of tertiary amines and arenesulfonyl azides with terminal alkynes. Sci China Chem, 2012, 55(2): 214–222
Karczmarzyk Z, Malinka W. Structural characterization of analgesic isothiazolopyridines of Mannich base type; X-ray analysis of 2-[(4-phenylpiperazin-1-yl)ethyl]-and 2-[(4-methylpiperazin-1-yl) methyl]-4,6-dimethylisothiazolo[5,4-b]pyridin-3(2H)-ones. J Mol Struct, 2008, 888: 160–167
Das U, Das S, Bandy B, Stables JP, Dimmock JP. N-Aroyl-3,5-bis(benzylidene)-4-piperidones: A novel class of antimycobacterial agents. Bioorg Med Chem, 2008, 16: 3602–3607
Ji L, Long QX, Yang DC, Xie JP. Identification of Mannich base as a novel inhibitor of mycoba-cterium tuberculosis isocitrate by high-throughput screening. Int J Biol Sci, 2011, 7: 376–382
Xu LY, Tang H, Dong JH, Wang WM, She ZZ. Synthesis and anti-inflammatory activity of the Mannich bases of 2-methyl-5-(E)-(2-methoxybenzylidene) cyclopentanone. Chin J Med Chem, 2002, 12: 1–4
Yang DC, Fan L, Liu HP, Song XL, Xu J, Zhao J, Deng Y, Li CB. Preparation of compounds with Mannich base structure and medical application for preventing and/or treating leukemia. Chinese Patent ZL200710092667.9, 2012-08-01
Joshi S, Khosla N, Khare D, Sharda R. Synthesis and in vitro study of novel Mannich bases as antibacterial agents. Bioorg Med Chem Lett, 2005, 15: 221–226
Zhou C, Wu G, Feng Y, Li Q, Su H, Mais D, Zhu Y, Li N, Deng Y, Yang DC, Wang MW. Discovery and biological characterization of a novel series of androgen receptor modulators. Br J Pharmacol, 2008, 154: 440–450
Wang H, Yan JF, Song XL, Fan L, Xu J, Zhou GM, Jiang L, Yang DC. Synthesis and antidiabetic performance of β-amino ketone containing nabumetone moiety. Bioorg Med Chem, 2012, 20: 2119–2130
Yang DC, Yan JF, Xu J, Ye F, Zhou ZW, Zhang WY, Fan L, Chen X. Synthesis and investigation on antidiabetic activity of 4-(1-aryl-3-oxo-5-phenylpentyl-amino) benzenesulfon-amide. Acta Pharm Sin, 2010, 45: 66–71
Zhang YX, Yan JF, Fan L, Zhang WY, Zhou ZW, Chen X, Su XY, Tang XM, Yang DC. Synthesis and preliminary evaluation of antidiabetic activity of 4-(3-(4-bromophenyl)-3-oxo-1-aryl-propylamino)-N-(5-methylisoxazol-3-yl) benzenesulfonamide. Acta Pharm Sin 2009, 44: 1244–1251
Xu J, Yan J. F, Fan L, Song XL, Tang XM, Yang DC. Synthesis and α-glucosidase inhibitory activity of N-(1,5-diaryl-3-pentone-1-yl)-4-aminobenzoic acid. Acta Pharm Sin, 2009, 44: 48–55
Zhou ZW, Yan JF, Tang XM, Zhang WY, Zhang YX, Chen X, Su XY, Yang DC. Synthesis and preliminary evaluation of antidiabetic activity for β-amino ketone containing isoxazole moiety. Chin J Org Chem, 2010, 30: 582–589
Sun J, Yang YS, Li W, Zhang YB, Wang XL, Tang JF, Zhu HL. Synthesis, biological evaluation and molecular docking studies of 1,3,4-thiadiazole derivatives containing 1,4-benzodioxan as potential antitumor agents. Bioorg Med Chem Lett, 2011, 21: 6116–6121
Prabhu PP, Shastry CS, Pande S, Pai A. Synthesis, characterization and biological evaluation of novel aryl benzothiazole derivatives. J Pharm Res, 2011, 4: 2209–2211
Qiu JY, Xu BX, Huang ZM, Pan WD, Cao PX, Liu CX, Hao XJ, Song BA, Liang GY. Synthesis and biological evaluation of Matijing-Su derivatives as potent anti-HBV agents. Bioorg Med Chem, 2011, 19: 5352–5360
Chen XF, Wu YB, Jin J, Wang RZ, Wang Y, Liu J. Design, synthesis of quinolinone acid-containing compounds with anti-HIV integrase activity. Acta Pharm Sin, 2010, 45: 263–267
Tang XM, Fan L, Yu HX, Liao YH, Yang DC. Facile synthesis of dipeptidomimetics of p-aminobenzoic acid and their antidiabetic activity. Chin J Org Chem, 2009, 29: 595–600
Mulongo G, Mbabazi J, Odongkara B, Twinomuhwezi H, Mpango GB. New biologically active compounds from 1, 3-diketones. Res J Chem Sci, 2011, 1: 102–108
Ammar Y, Mohamed Y, EI-Sharief A, EI-Gaby M, Abbas S. Reactivity of 2, 3-pyridine dicarboxylic anhydride towards some nitrogen nucleophilic reagents: Synthesis and antimicrobial evaluation of some pyridine carboxamide and pyrrolo[3,4-B]pyridine-5,7-dione derivatives. Chem Sci J, 2011, 16: 1–11
Feng J, Guo YS, Lu Y, Guo ZR. Design, virtual screening and synthesis of PPAR agonists. Acta Chim Sin, 2004, 62: 1544–1550
Hedner T, Samulesson O, Wahrborg P, Wadenvik H, Ung K, Ekbom A. Nabumetone therapeutic use and safety profile in the management of osteoarthritis and rheumatoid arthritis. Drug, 2004, 64: 2315–2343
Zou JH, Yi L. The Mannich reaction among acetophenone, p-methoxybenzaldehyde and arylamines. J Southwest Chin Norm Univ (Nat Sci), 1991, 16: 66–70
Azizi N, Torkiyan L, Saidi MR. Highly efficient one-pot three-component Mannich reaction in water catalyzed by heteropoly acids. Org Lett, 2006, 8: 2079–2082
Ma XZ, Yi LN, Liu YX, Mei WJ, She ZZ. Synthesis and antitumor activities of 2-(E)-(4-cyclopentyloxy-3-methylbenzyl idene) cyclop-entanone arylamine Mannich bases. Chin J Med Chem, 2006, 16: 144–149
Li G, Li ZY, Wu CT. Michael addition of monoazabenzo-15-C-5 with acrylic esters and amides. Chin J Org Chem, 1998, 18: 462–464
Chen GX, Xu XJ, Liu LJ. Mannich reaction with arylamine as the aminecomponent (II). Chem J Chin Univ, 1982, 3: 83–90
Xiao J, Wu YR. The preparation of N-Mannich base and its exchange reaction with arylketones. J Beijing Norm Univ, 1996, 32: 367–369
Takaya J, Kagoshima H, Akiyama T. Mannich-type reaction with trifluoromethylated N, O-hemiacetal: Facile preparation of β-amino-β-trifluoromethyl carbonyl compounds. Org Lett, 2000, 2: 1577–1579
Xu XJ, Chen GX. The Mannich reaction with arylamines. Acta Chimica Sin, 1982, 40: 463-467
Yi L, Zhou JH, Xu XJ. The Mannich reaction among aromatic ketone, aromatic aldehyde and arylamines. Chin Chem, 1991, 5: 20–21
Yi L, Zhou JH, Lei HS, Lin XM, Zhang MX. The Mannich reaction of cyclic ketones, aromatic aldehydes and aromatic amines. Org Prep Proced Int, 1991, 23: 673–676
Yang DC. The Mannich reaction of 4-phenyl-2-butanone with aromatic aldehydes and aromatic amines. J Southwest Chin Norm Univ (Nat Sci), 1996, 21: 354–359
Yang DC, Zhang GL, Yang Y, Zhong YG. The Mannich reaction of 4-methylacetophenone with aromatic aldehydes and aromatic amines. Chem J Chin Univ, 2000, 21: 1694–1696
Zhang K, Yan JF, Tang XM, Liu HP, Fan L, Zhou GM, Yang DC. Synthesis of novel β-aminoalcohols containing nabumetone moiety with potential antidiabetic activity. Acta Pharm Sin, 2011, 46: 412–421
Yang DC, Yan JF, Song XL, Zhou ZW, Tang XM, Chen X, Fan L. Synthesis and preliminary evaluation of antidiabetic activity of 4-[3-(4-bromophenyl)-3-oxo-1-arylpropylamino] benzenesulfonamide. Acta Chim Sin, 2010, 68: 515–522
Zhang YX, Yan JF, Fan L, Zhang WY, Su XY, Chen X, Tang XM, Zhou ZW, Yang DC. Synthesis and preliminary evaluation of antidiabetic activity of 4-(3-(4-hydroxyphenyl)-3-oxo-1-aryl-propyl-amino)-N-(5-methylisoxazol-3-yl)benzenesulfonamide. Chin J Applied Chem, 2010, 27: 1026–1031
Song XL, Yan JF, Fan L, Xu J, Zhou ZW, Yang DC. Synthesis and preliminary evaluation of antidiabetic activity of 4-(1-aryl-3-aryl/arylalkyl-3-oxopropylamino)-N-(5-methyl-3-isoxazolyl) benzenesulfonamide. Chin J Org Chem, 2009, 29: 606–613
Tang XM, Yan JF, Zhang YX, Zhang WY, Su XY, Chen X, Zhou ZW, Yang DC. Synthesis and preliminary study on α-glucosidase inhibitory activity of 4-[3-(4-bromophenyl)-3-oxo-1-arylpropylamino]-N-(pyrimidin-2-yl)benzenesulfonamide. Chin J Org Chem, 2009, 29: 1790–1798
Li QL, Yang DC, Yan JF, Fan L, Chen X, Liu HP, Zhang WY, Song XL, Yan YH, Xu J, Zhang J. Preparation of β-aminoketone (alcohol) derivatives as PPAR agonists or insulin sensitizers. Chinese Patent Application: 200910258861.200910258861.9; 2009
Yang DC, Yan JF, Fan L, Chen X, Xu J, Zhang WY, Song XL, Ye F, Liu HP, Jiang HW, Zhou ZW, Tang XM, Zhang YX, Li TJ, Su XY. Preparation of β-aminoketone derivatives for treating diabetic mellitus. Chinese Patent Application 200810237001.200810237001.2; 2008
Wang MW, Deng Y, Zhou CH, Yang DC, Hui X, Su YR, Gao J. 3-Phenyl-3-(4-nitrobenzeneamino)-1-(4-chlorophenyl)-1-acetone as non-steridesandrogen receptor moderator. Chinese Patent ZL200910259690.1, 2011-04-27
Yang DC, Xie JP, Fan L, Yi L, Tang XM, Zhou ZW, Zhang YX, Su XY. Application of β-amino ketone compounds to prepare medical agents for inhibiting isocitrate lyase. Chinese Patent Application: 200910104544.1; 2009
Rao RR, Tiwari AK, Reddy P, Babu KS, Suresh G, Ali AZ, Madhusudana K, Agawane SB, Badrinarayan P, Sastry GN, Rao JM. Synthesis of antihyperglycemic, α-glucosidase inhibitory, and DPPH free radical scavenging furanochalcones. Med Chem Res, 2012, 6: 760–774
Tomich CH, Silva PD, Carvalho I, Taft CA. Homology modeling and molecular interaction field studies of α-glucosidases as a guide to structure-based design of novel proposed anti-HIV inhibitors. J Comput Aided Mol Des, 2005, 19: 83–92
Lazar C, Durantel D, Macovei A, Zitzmann N, Zoulim F, Dwek RA, Branza-Nichita N. Treatment of hepatitis B virus-infected cells with a-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antiviral Res, 2007, 76: 30–37
Hakamata W, Kurihara M, Okuda H, Nishio T, Oku T. Design and screening strategies for α-glucosidase inhibitors based on enzymological information. Curr Top Med Chem, 2009, 9: 3–12
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tang, G., Yan, J., Fan, L. et al. Synthesis of novel β-amino ketones containing a p-aminobenzoic acid moiety and evaluation of their antidiabetic activities. Sci. China Chem. 56, 490–504 (2013). https://doi.org/10.1007/s11426-012-4816-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4816-2