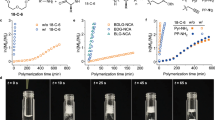
Abstract
In polymerization of N-carboxyanhydride-L-α-arginine (L-Arg-NCA) in H2O, nucleophilic reaction of guanidine group with the carbonyl group of L-Arg-NCA leads to quick intramolecular rearrangement, yielding a 6-membered ring intermediate 1-amidino-3-amino-2-piperidone, which is either elongated by another L-Arg-NCA yielding arginyl-1-amidino-3-amino-2-piperidone or hydrolyzed to L-α-arginine. The oligoarginines are formed mainly through hydrolysis of arginyl-1-amidino-3-amino-2-piperidones. This is a unique pathway in polymerization of L-Arg-NCA with regard to the usual pathway of elongations by reaction of N-carboxyanhydride-L-α-amino acid with L-α-amino acid or oligopeptides.
Similar content being viewed by others
References
Stahmann M. Polyamino Acids, Polypeptides and Proteins. Madison, Wisconsin: Univ Wisconsin Press, 1962
Katchelski E, Sela M, Silman H I, Berger A. Polyamino Acids as Protein Models. The Proteins. Vol II. 2nd ed. New York: Academic Press, 1964. 405–581
Kricheldorf H R. α-Aminoacid-N-Carboxy-Anhydrides and Related Heterocycles: Synthesis, Properties, Peptide Synthesis. Polymerization. Berlin: Springer-Verlag, 1987. 1–209
Kricheldorf H R. Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew Chem Int Ed, 2006, 45: 5752–5784
Ehler K W. Reactions of carbamylimidazoles with nucleophiles-An example of an intramolecular acyl transfer reaction. J Org Chem, 1976, 41: 3041–3042
Brack A. Selective emergence and survival of early polypeptides in water. Orig Life Evol Biosph, 1987, 17: 367–379
Hill R., Orgel L E. Oligomerization of negative-charged amino acids by carbonyldiimidazole. Origins Life Evol Biosph, 1996, 26: 539–545
Blocher M, Liu D, Walde P, Luisi P L. Liposome-assisted selective polycondensation of α-amino acids and peptides. Macromolecules, 1999, 32: 7332–7334
Pascal R, Boiteau L, Commeyras A. From the prebiotic synthesis of α-amino acids towards a primitive translation apparatus for the synthesis of peptides. Top Curr Chem, 2005, 259: 69–122
Plasson R, Biron J P, Cottet H, Commeyras A, Taillades J. Kinetic study of the polymerization of α-aminoacid N-carboxyanhydrides in aqueous solution using capillary electrophoresis. J Chromatograph A, 2002, 952: 239–248
Hitz T, Luisi P L. Spontaneous onset of homochirality in oligopeptide chains generated in the polymerization of N-carboxyanhydride amino acids in water. Origins Life Evol Biosph, 2004, 34: 93–110
Kricheldorf H R. Polypeptides. In: Penczek S, ed. Models of Biopolymers by Ring-Opening Polymerization. Boca Raton, Florida: CRC Press, 1990. 1–132
Zervas L, Winitz M, Greenstein J P. Studies on arginine peptides. I. Intermediates in the synthesis of N-terminal and C-terminal arginine peptides. J Org Chem. 1957, 22: 1515–1521
Hayakawa T, Kondo Y, Yamamoto H, Murakami Y. The synthesis of poly-L-arginine hydrobromide and copolymers of L-arginine and other amino acids. Bull Chem Soc Jpn, 1969, 42: 479–482
Hayakawa T, Windsor C R, Fox S W. Copolymerization of the Leuchs anhydrides of the eighteen amino acids common to protein. Arch Biochem Biophys, 1967, 118: 265–272
Zhang L S, Tam J P. Lactone and lactam library synthesis by silver ion-assisted orthogonal cyclization of unpro-tected peptides. J Am Chem Soc, 1999, 121: 3311–3320
Xin L, Ma Y L, Liu Y N, Yan Q, Wang K J. Sodium chloride enhanced oligomerization of L-arginine. Biopolymers, 2006, 81: 1–7
Ren J, Xin L, Liu Y N, Wang K J. Copolymerization of mixed L-alpha-arginine with L-alpha-glutamic acid. Macromolecules, 2008, 41: 1996–2002
Bartlett P D, Jones R H. A kinetic study of the Leuchs anhydrides in aqueous solution. J Am Chem Soc, 1957, 79: 2153–2159
Ehler K W, Orgel L E. N,N′-carbonyldiimidazole-induced peptide formation in aqueous solution. Biochim Biophys Acta, 1976, 434: 233–243
Liu R H, Orgel L E. Polymerization of beta-amino acids in aqueous solution. Origins Life Evol Biosph, 1998, 28: 47–60
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the Special Research Project of Beijing Scientific and Technical Committee (Grant No. Z00063002040191) and the Natural Basic Research Program of China (Grant No. 2007CB935901)
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xin, L., Ren, J., Xiang, J. et al. 6-Membered ring intermediates in polymerization of N-carboxyanhydride-L-α-arginine in H2O. Sci. China Ser. B-Chem. 52, 1220–1226 (2009). https://doi.org/10.1007/s11426-009-0112-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0112-1