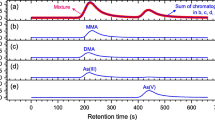
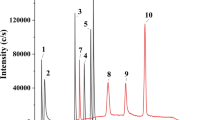
Abstract
Purpose
Arsenic, especially inorganic trivalent species, is one of the most important poisons in the field of forensic toxicology. There are many reports on the speciation analysis of arsenic species in biological specimens by liquid chromatography–inductively coupled plasma-mass spectrometry (LC–ICP-MS). The aim of this study was to develop a rapid and robust analytical method for speciation/quantitative analysis of arsenic species in serum by LC–ICP-tandem mass spectrometry (MS/MS).
Methods
An analytical method for arsenous acid and its metabolites, dimethylarsinic acid, monomethylarsonic acid, and arsenic acid in serum was tested through the analysis of serum samples by an LC–ICP-MS/MS system consisting of different anion exchange columns and different mobile phases. Rapid pretreatment of serum samples by ultrafiltration was also tested.
Results
Robust speciation/quantitative analysis of arsenic in serum samples with LC–ICP-MS/MS was achieved by using a mildly acidic mobile phase. The limits of detection for the four arsenic species were in the range 0.19–0.68 ngAs/mL, and the well-known interference by argon chloride ion was removed by the MS/MS apparatus. This method was precise enough for quantitative analysis of four arsenic compounds in serum (0.24–3.68% precision; 97.0–104% accuracy; and 101–112% recovery for all analytes at 5 and 50 ngAs/mL). The total analytical time was 30 min (20-min pretreatment and 10-min analysis), and multiple serum samples could be pretreated simultaneously.
Conclusions
A rapid, sensitive, interference-free and robust speciation/quantitative analysis of toxic arsenous acid and related metabolites in serum by LC–ICP-MS/MS was developed. To our knowledge, this is the first report to use LC–ICP-MS/MS for analysis of arsenic species in human blood/serum samples.





Similar content being viewed by others
References
Benramdane L, Bressolle F, Vallon JJ (1999) Arsenic speciation in humans and food products: a review. J Chromatogr Sci 37:330–344
Delafiori J, Ring G, Furey A (2016) Clinical applications of HPLC-ICP-MS element speciation: a review. Talanta 153:306–331
Marcinkowska M, Barałkiewicz D (2016) Multielemental speciation analysis by advanced hyphenated technique—HPLC/ICP-MS: a review. Talanta 161:177–204
Okina M, Yoshida K, Kuroda K, Wanibuchi H, Fukushima S, Endo G (2004) Determination of trivalent methylated arsenicals in rat urine by liquid chromatography-inductively coupled plasma mass spectrometry after solvent extraction. J Chromatogr B 799:209–215
Heitland P, Köster HD (2009) Comparison of different medical cases in urinary arsenic speciation by fast HPLC-ICP-MS. Int J Hyg Environ Health 212:432–438
Verdon CP, Caldwell KL, Fresquez MR, Jones RL (2009) Determination of seven arsenic compounds in urine by HPLC-ICP-DRC-MS: a CDC population biomonitoring method. Anal Bioanal Chem 393:939–947
Serrano IN, Ballesteros MTL, Pacheco SSF, Álvarez SI, Colón JLL (2016) Total and speciated urinary arsenic levels in the Spanish population. Sci Total Environ 571:164–171
Heitland H, Blohm M, Breuer C, Brinkert F, Achilles EG, Pukite I, Köster HD (2017) Application of ICP-MS and HPLC-ICP-MS for diagnosis and therapy of a severe intoxication with hexavalent chromium and inorganic arsenic. J Trace Elem Med Biol 41:36–40
Wang D, Shimoda Y, Wang S, Wang Z, Liu J, Liu X, Jin H, Gao F, Tong J, Yamanaka K, Zhang J, An Y (2017) Total arsenic and speciation analysis of saliva and urine samples from individuals living in a chronic arsenicosis area in China. Environ Health Prev Med 22:45. https://doi.org/10.1186/s12199-017-0652-5
Carioni VMO, McElroy JA, Guthrie JM, Ngwenyama RA, Brockman JD (2017) Fast and reliable method for As speciation in urine samples containing low levels of As by LC-ICP-MS: focus on epidemiological studies. Talanta 165:76–83
Yuan C, Lu X, Oro N, Wang Z, Xia Y, Wade TJ, Mumford J, Le XC (2008) Arsenic speciation analysis in human saliva. Clin Chem 54:163–171
Kintz P, Ginet M, Marques N, Cirimele V (2007) Arsenic speciation of two specimens of Napoleon’s hair. Forensic Sci Int 170:204–206
Contreras-Acuña M, García-Barrera T, García-Sevillano MA, Gómez-Ariza JL (2014) Arsenic metabolites in human serum and urine after seafood (Anemonia sulcata) consumption and bioaccessibility assessment using liquid chromatography coupled to inorganic and organic mass spectrometry. Microchem J 112:56–64
Fukai Y, Hirata M, Ueno M, Ichikawa N, Kobayashi H, Saito H, Sakurai T, Kinoshita K, Kaise T, Ohta S (2006) Clinical pharmacokinetic study of arsenic trioxide in an acute promyelocytic leukemia (APL) patient: speciation of arsenic metabolites in serum and urine. Biol Pharm Bull 29:1022–1027
Ito K, Goessler W, Gürleyük H, Wels B, Palmer CD, Verostek MF, Parsons PJ (2011) An interlaboratory study of arsenic speciation analysis of whole blood. J Anal At Spectrom 26:1740–1745
Araujo-Barbosa U, Peña-Vazquez E, Barciela-Alonso MC, Costa Ferreira SL, Pinto Dos Santos AM, Bermejo-Barrera P (2017) Simultaneous determination and speciation analysis of arsenic and chromium in iron supplements used for iron-deficiency anemia treatment by HPLC-ICP-MS. Talanta 170:523–529
Yigzaw Y, Hinckley P, Hewig A, Vedantham G (2009) Ion exchange chromatography of proteins and clearance of aggregates. Current Pharm Biotechnol 10:421–426
Goheen SC, Gibbins BM (2000) Protein losses in ion-exchange and hydrophobic interaction high-performance liquid chromatography. J Chromatogr A 890:73–80
Muca R, Marek W, Żurawski M, Piątkowski W, Antos D (2017) Effect of mass overloading on binding and elution of unstable proteins in hydrophobic interaction chromatography. J Chromatogr A 1492:79–88
Bandura DR, Baranov VI, Tanner SD (2002) Detection of ultratrace phosphorus and sulfur by quadrupole ICPMS with dynamic reaction cell. Anal Chem 74:1497–1502
Schaumlöffel D, Giusti P, Preud’Homme H, Szpunar J, Łobiński R (2007) Precolumn isotope dilution analysis in nanoHPLC-ICPMS for absolute quantification of sulfur-containing peptides. Anal Chem 79:2859–2868
Zinn N, Krüger R, Leonhard P, Bettmer J (2008) μLC coupled to ICP–SFMS with post-column isotope dilution analysis of sulfur for absolute protein quantification. Anal Bioanal Chem 391:537–543
Šlejkovec Z, Podgornik H, Černelč P, Falnoga I (2016) Exceptions in patterns of arsenic compounds in urine of acute promyelocytic leukaemia patients treated with As2O3. Biometals 29:107–118
Šlejkovec Z, Falnoga I, Goessler W, van Elteren JT, Raml R, Podgornik H, Černelč P (2008) Analytical artefacts in the speciation of arsenic in clinical samples. Anal Chim Acta 607:83–91
Guo M, Wang W, Hai X, Zhou J (2017) HPLC-HG-AFS determination of arsenic species in acute promyelocytic leukemia (APL) plasma and blood cells. J Pharm Biomed Anal 145:356–363
Chen YW, Belzile N (2010) High performance liquid chromatography coupled to atomic fluorescence spectrometry for the speciation of the hydride and chemical vapour-forming elements As, Se, Sb and Hg: a critical review. Anal Chim Acta 671:9–26
Schmidt L, Landero JA, Santos RF, Mesko MF, Mello PA, Flores EMM, Caruso JA (2017) Arsenic speciation in seafood by LC-ICP-MS/MS: method development and influence of culinary treatment. J Anal At Spectrom 32:1490–1499
Guimarães D, Roberts AA, Tehrani MW, Huang R, Smieska L, Woll AR, Lin S, Parsons PJ (2018) Characterization of arsenic in dried baby shrimp (Acetes sp.) using synchrotron-based X-ray spectrometry and LC coupled to ICP-MS/MS. J Anal At Spectrom 33:1616–1630
Schmidt L, Landero JA, La Rosa Novo D, Duarte FA, Mesko MF, Caruso JA, Flores EMM (2018) A feasible method for As speciation in several types of seafood by LC-ICP-MS/MS. Food Chem 255:340–347
Japanese Standards Association (2006) High frequency plasma mass spectrometry general notice, K0133. In: JIS Handbook. Japanese Standards Association, Tokyo, pp 371–388 (in Japanese)
Higashikawa Y, Kazui Y, Suzuki S, Ohtsuru O (2008) Arsenic speciation of arsine-exposed blood samples by high-performance liquid chromatography-inductively coupled plasma mass spectrometry and As-adduct, a possible indicator of AsH3 exposure. J Anal Toxicol 32:344–348
Tan SH, Horlick G (1986) Background spectral features in inductively coupled plasma/mass spectrometry. Appl Spectrosc 40:445–460
Kaise T (2002) Speciation of arsenic compounds in biological samples by high performance liquid chromatography-inductively coupled plasma mass spectrometry system. J Plasma Fusion Res 78:646–652 (in Japanese with English abstract)
Ohwaki S, Matsuoka M, Sato T, Kikukawa K, Kobayashi M, Kaneko S (2018) Arsenic speciation analysis in seaweed and rice flour samples by ion chromatography combined with inductively coupled plasma-mass spectrometry. Studies Sci Technol 7:69–74 (in Japanese)
Yoshinaga J, Chatterjee A, Shibata Y, Morita M, Edmonds JS (2000) Human urine certified reference material for arsenic speciation. Clin Chem 46:1781–1786
Oliver LK (2009) Laboratory assessment of exposure to neurotoxic agents. In: Dobbs M (ed) Clinical neurotoxicology. Saunders, Philadelphia, pp 213–221
Acknowledgements
This work was supported by the research project “R&D of chemical examination method by the application of highly effective instruments for elemental analysis to the discrimination of evidential material” by National Research Institute of Police Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The coauthor Yasuo Seto is a Chief Editor of the Forensic Toxicology, but was not involved in the peer-reviewing of this article. Other authors declare no competing interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazui, Y., Ohta, H., Watanabe, D. et al. Rapid and robust speciation/quantitative analysis of arsenous acid and related metabolites in serum by liquid chromatography–inductively coupled plasma-tandem mass spectrometry. Forensic Toxicol 37, 424–431 (2019). https://doi.org/10.1007/s11419-019-00479-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-019-00479-w