Abstract
Comprehensive chemical characterization for two cathinone derivatives, N-ethyl-2-amino-1-phenylpropan-1-one (ethcathinone) hydrochloride and N-ethyl-2-amino-1-(4-chlorophenyl)-propan-1-one (4-chloroethcathinone, 4-CEC) hydrochloride, in material seized by drug enforcement agencies was performed by nuclear magnetic resonance (NMR) spectroscopy, infrared spectroscopy, gas chromatography–mass spectrometry in positive electron ionization mode, liquid chromatography–mass spectrometry in positive electrospray ionization mode and X-ray crystallography. The examined samples of these two compounds proved to be very pure for ethcathinone and mixed with very small quantities of other substances for 4-CEC by NMR spectroscopy and mass spectrometry. X-ray crystallographic studies confirmed the occurrence of both compounds as racemic mixtures. These spectroscopic and crystallographic data seem very useful for their identification. Especially for 4-CEC, this is the first description on its spectroscopic characterization in a scientific context to our knowledge.
Similar content being viewed by others
Introduction
Synthetic cathinones are based on naturally occurring cathinone, one of the psychoactive compounds present in khat (Catha edulis), a plant growing in the Arabian Peninsula, Ethiopia and Somalia, as well as cultivated in other East African countries. The plant is variably called Arabian Tea, Abyssinian Tea, Chat Tree, Khat or Cafta. Its leaves contain ca. 1% alkaloids. The main ones are: cathinone (2-amino-1-phenylopropan-1-one, α-aminopropiophenone), l-ephedrine and cathin [(1s, 2s)-2-amino-1-phenylpropan-1-ol, (+)-nor-ψ-ephedrine].
Synthetic cathinones first appeared on the European illicit drug market in 2005 and today they make up one of the largest categories of novel psychoactive substances (NPSs) as over 80 synthetic cathinone derivatives were detected via the EU Early Warning System between 2005 and 2014 [1]. Cathinone derivatives are claimed to have effects similar to those of cocaine, amphetamine or MDMA (ecstasy), but little is known about their toxicity and pharmacokinetic properties. Synthetic cathinones most often are sold as powders or tablets through physical retail shops or via the Internet labeled as ‘plant feeders’, ‘plant food’, ‘research chemical’, or ‘bath salts’. Various products are labeled with such warnings as ‘not for human consumption' or ‘not tested for hazards or toxicity'. The availability of high-quality and pure synthetic cathinones has sometimes been reported as offering direct competition to low-quality and relatively more expensive established drugs. The most common administration routes include insufflation (snorting) and oral ingestion of capsules, tablets or powder wrapped in a cigarette paper (so-called ‘bombing’). Rectal insertion and intravenous, subcutaneous and intramuscular injections were also reported [2–4].
Ethcathinone (compound 1) was one of the most common NPSs on the illegal drug market in Poland between 2012 and 2014 [5, 6]. In Poland, since July 2015, ethcathinone has the status of an controlled substance. Compound 1 (ethcathinone) was subjected to pharmacological studies because it is a metabolite of diethylpropion (N,N-diethyl-2-amino-1-phenylpropan-1-one), which proved to be more potent than its parent compound. This led to the conclusion that it is the metabolite, and not the starting diethylpropion, that causes the specific anorectic effect [7]. Compound 2 (4-chloroethcathinone, 4-CEC) had been briefly studied in terms of its potential anorectic effect [8]. Its current availability on the illegal drug market makes it probable that this compound will be taken off the register of medical drug candidates.
Only the 1H nuclear magnetic resonance (NMR) [7] and Fourier transform infrared (FTIR) [9] spectra of compound 1 have been published. Also a few spectroscopic data for compound 2 are available on the Internet only [10]. Both compounds are similar to another synthetic cathinone used recreationally and described earlier, i.e., N-ethyl-2-amino-1-(4-methylphenyl)propan-1-one hydrochloride (short name: 4-MEC) [11]. Compound 1 was examined chromatographically [9] and some mass spectrometry (MS) data were reported for this cathinone [9, 12, 13].
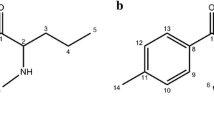
Both compounds can be synthesized [12, 14, 15] starting from suitable racemic mixtures of 2-bromopropiophenones with ethylamine. The products of this reaction are largely racemic mixtures of ethcathinone hydrochloride (compound 1; in case of using 2-bromopropiophenone) or 4-chloroethcathinone (4-CEC) hydrochloride (compound 2; in case of using 4′-chloro-2-bromopropiophenone) (Fig. 1).
Samples of the compounds 1 and 2 analyzed here by spectroscopy and crystallography exclusively originated from material seized by drug enforcement agencies. They either did not contain additional substances (in case of compound 1), or were mixed with small quantities of other substances which allowed, however, mechanical separation of thick crystals (compound 2) of very good purity.
In this study, we report the spectroscopic characteristics and crystallographic structures of compounds 1 and 2. To our knowledge, such data are unavailable, especially for compound 2.
Materials and methods
Compounds 1 and 2 were provided by drug enforcement agencies either in pure form (compound 1), or as a mixture of crystals (compound 2) with less than 1% of ingredients (according to NMR data). Crystals of compound 1 suitable for crystallography were obtained by very slow evaporation of solutions used to study this compound by NMR spectroscopy. The pure compound 2 crystals were mechanically separated, recrystallized from a mixture of dichloromethane and acetone, and used in this study.
1H NMR spectra were recorded in D2O or dimethyl sulfoxide (DMSO)-d 6 using a Varian spectrometer (400 MHz) (Varian, Palo Alto, CA, USA). The peaks were referenced to the residual H2O (4.63 ppm) and DMSO (2.49 and 39.5 ppm) resonances in 1H and 13C NMR. Infrared (IR) spectra were recorded on a Bio-Rad FTS-600 (Bio-Rad Laboratories, Hercules, CA, USA) with the samples in the form of KBr pellets. Liquid chromatography–mass spectrometry (LC–MS) analysis of samples was performed on a Thermo Scientific TSQ Quantum Access Max LC–MS operating in positive electrospray ionization (ESI) mode (Thermo Scientific, Waltham, MA, USA). Separation was achieved on a BDS Hypersil C18 (150 × 2.1 mm, 5 μm) column (Thermo Scientific) maintained at 25 °C. The mobile phase A was water, which contained 0.2% formic acid and 2 mM of ammonium formate, and phase B was acetonitrile with 0.2% formic acid and 2 mM of ammonium formate. The gradient program was applied. The operational parameters of the ESI source were as follows: vaporizing temperature 350 °C; pressure of the nebulizing gas 40 psi; capillary potential 3500 V. Gas chromatography–mass spectrometry (GC–MS) analyses were performed using a gas chromatograph (TRACE 1300 gas chromatograph) coupled to a mass spectrometer (ISQ LT) equipped with a quadruple mass analyzer (Thermo Scientific). The injector was maintained at 280 °C. Sample injection was in splitless mode. Sample component separation was conducted on an RTX-5 capillary column (30-m length, 0.25-mm inner diameter, 0.25-µm film thickness; Restek, Bellefonte, PA, USA). Helium was used as a carrier gas at the flow rate of 1.0 mL/min. The temperature program consisted of three segments: the initial column temperature (75 °C) was maintained for 1 min, then was increased linearly at 25 °C/min up to 280 °C, and maintained for 20.8 min. The mass detector was set to positive electron ionization (EI) mode, and the electron beam energy was 70 eV. The mass detector was operating in a full scan mode in the range of m/z 40–450.
The single crystal X-ray experiments were performed at room temperature. The data were collected using a SuperNova kappa diffractometer with an Atlas charge coupled device (CCD) detector and a Xcalibur diffractometer with a Sapphire3 CCD detector (Oxford Diffraction Ltd., Yarnton, England). For the integration of the collected data, the CrysAlis RED software (version 1.171.32.29; Agilent Technologies, Santa Clara, CA, USA) was used. The solving and refining procedures were similar for both compounds. The structures were solved using direct methods with the SHELXS97 software and the solutions were refined using the SHELXL-2014/7 program [16]. CCDC 1481895 (1) and 1481896 (2) contain supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via: www.ccdc.cam.ac.uk/data_request/cif.
Results and discussion
NMR spectra
Samples of both compounds received for analysis were examined by 1H and 13C NMR and IR spectroscopy, and ESI-MS, which confirmed that they were cathinone derivatives. Figures 2 and 3 show NMR spectra of compounds 1 and 2, respectively. They fully confirm the predicted structures of the examined species. At the same time, they demonstrate that the examined substances are nearly pure cathinones. No other signals were detected which would imply the presence of other impurities in the examined sample of compound 1. The 1H NMR spectrum of compound 2 showed signals at 7.95, 7.40, 5.05, 3.45, 3.16, 2.48, 2.06 and 1.18 ppm of very low intensities reflecting some undefined impurities (approximate content of impurities <1%; Fig. 3, upper panel). The N–H protons in the hydrochloride salt of compound 2 are diastereotopic (chemically inequivalent). These protons were observed as two broad singlets at δ = 9.74 and 9.22 ppm but only in DMSO-d 6 solution (Fig. 3, upper panel). In D2O or CD3OD solutions, these signals were not observed (Fig. 2, upper panel). The 1H NMR spectra of both compounds confirmed the presence of a substituted benzene ring, which was mono-substituted (compound 1) and di-substituted (para, compound 2). The methinic protons in compounds 1 and 2 appeared as quintets at 5.07 and 5.21 ppm, respectively (see below). The N-ethyl side chains yielded two multiplets for methylene protons centered at 3.05 and 3.15 ppm for compound 1, and two broad multiplets (like broad singlets) centered at 2.93 and 3.05 ppm for compound 2. Terminal methyl groups yielded triplets centered at 1.27 and 1.28, for compounds 1 and 2, respectively.
The 13C NMR spectra displayed carbonyl resonance at 197.6 and 195.9 ppm for compounds 1 and 2, respectively (Figs. 2,3, lower panels, also see below). The six aromatic carbons appearing as four signals for both compounds showed greater differences because they reflect chemical and structural differences. The methylene carbons of N-ethyl groups were found at 41.3 and 40.8 ppm for compounds 1 and 2, respectively. The methinic carbons resonated at 58.0 and 57.2 ppm for compounds 1 and 2, respectively. The up-field two signals in both spectra correspond to both methyl carbons of each compound.
Compound 1 (ethcathinone hydrochloride)
1H NMR (D2O), δ (ppm): 7.94 (d, 2H), 7.69 (t, 1H), 7.53 (t, 2H), 5.07 (q, 1H), 3.15 (m, 1H), 3.05 (m, 1H), 1.52 (d, 3H), 1.27 (t, 3H).
13C NMR (D2O), δ (ppm): 197.6, 135.4, 132.3, 129.3, 128.9, 58.0, 41.3, 15.5, 10.7.
Compound 2 (4-CEC hydrochloride)
1H NMR (DMSO-d 6), δ (ppm): 9.74 (bs, 1H), 9.22 (bs, 1H), 8.09 (d, 2H), 7.68 (d, 2H), 5.21 (q, 1H), 3.05 (bs, 1H), 2.93 (bs, 1H), 1.47 (d, 3H), 1.28 (t, 3H).
13C NMR (DMSO-d 6), δ (ppm): 195.9, 140.1, 132.1, 131.2, 129.8, 57.2, 40.8, 15.9, 11.6.
IR spectra
Only IR spectra of compound 1 were previously published [9]. The IR spectra showed strong C=O absorption bands at 1694 and 1686 cm−1 for compounds 1 and 2, respectively. The shift of the C=O absorption band for one compound relative to the other is a result of a substituent in a para position of the aromatic ring in compound 2. Spectra of both compounds showed characteristic bands associated with their salt forms. These included a broad pattern from 2300 to 3100 cm−1 corresponding to amine salt absorption bands and combination of aromatic and aliphatic C–H stretches. The main NH2 + stretch absorption for both compounds appeared at 2694 cm−1. The aromatic C=C ring stretch vibrations appeared at 1597 and ca. 1590 cm−1 for compounds 1 and 2, respectively. A strong band at 839 cm−1 in the spectrum of compound 2 was characteristic for two adjacent free hydrogen atoms in an aromatic para-di-substituted ring (aromatic C–H out-of-plane bend). Two bands at 768 and 698 cm−1 in compound 1 were characteristic for an aromatic C–H out-of-plane bend of a mono-substituted benzene ring. All other absorption peaks are listed below.
Compound 1 (ethcathinone hydrochloride)
IR (KBr; cm−1): 3417, 2934, 2803, 2742, 2480, 1694, 1597, 1439, 1387, 1338, 1314, 1237, 1194, 1128, 1105, 1025, 978, 861, 792, 768, 698, 684, 657, 537.
Compound 2 (4-CEC hydrochloride)
IR (KBr; cm−1): 3530, 3430, 2983, 2795, 2694, 2447, 1687, 1590sh, 1554, 1462, 1392, 1292, 1234, 1164, 1094, 1051, 974, 919, 839, 794, 748, 684, 479.
GC–MS and ESI-MS spectrometry
Figure 4 shows the EI mass spectra of compounds 1 and 2. Figure 5 shows EI fragmentation pathways of compound 1. For compound 2, fragmentation pathways was identical (with respect to the Cl substituent). The molecular ions for both compounds were not visible. Base peaks for compounds 1 and 2 at m/z 72 probably resulted from formation of N-ethylethano-1-iminium cation via the initiated benzoyl unit cleavage and by the loss of a hydrogen molecule. For compound 1, after cleavage of the β-ketone unit, the benzoyl cation at m/z 105 was formed with subsequent loss of a CO molecule to form a phenyl cation at m/z 77. Similarly, for compound 2, the same process led to the 4-chlorobenzoyl cation at m/z 139 and the next 4-chlorophenyl cation at m/z 111. These types of fragmentation are consistent with previously reported data [9, 17–19].
Figure 6a shows the ESI mass spectrum of compound 1 upon HPLC–MS analysis. A base peak appeared at m/z 178.16 in good accordance with the calculated value for [M + H]+ [calc. for M (C11H15NO); m/z 178.12]. Figure 6b shows the ESI mass spectrum of compound 2. Base peaks appeared at m/z 212.09 and 214.09 (for isotopic 37Cl atom). Both peaks were in good accordance with calculated values for [M + H]+ ions [calc. for M(C11H14ClNO); m/z 212.08 and 214.08 for 35Cl and 37Cl, respectively]. Isomer ratio occurring in nature was ca. 76/34 (35Cl/37Cl) which is almost ideally reflected by the intensities of both basic peaks.
The elimination of one water molecule from the protonated molecular ion is very characteristic for cathinones, especially in the tandem mode [19]. For both described compounds, this phenomenon appeared in MS1 spectra. In Fig. 7, we present proposed fragmentation pathways for ESI in MS2, MS3 and MS4 modes for compound 1 and compound 2 as well.
X-ray studies
Both hydrochloride salts crystallize in a monoclinic (P21/c) space group. The molecular structures of both compounds are shown in Fig. 8. Packing diagrams for both compounds are shown in Fig. 9. Crystal data and structure refinement for compounds 1 and 2 are presented in Table 1. The ring systems in both compounds were planar. All distances and angles in the molecular structures were normal. Hydrogen bonds in compounds 1 and 2 are listed in Tables 2 and 3, respectively. Selected geometric parameters for compounds 1 and 2 are presented in Table 4.
Compound 1
Both compounds occurred in the examined crystals as paired enantiomers. The pairs were mutually linked by hydrogen bonds formed with chloride ions occurring between these molecules. For compound 1, all short contacts were linked to methyl groups (in position 2, and in ethyl group) and chloride ions freely located between molecules of the compound as well as with a ketone group of oxygen atoms. Chloride ions were placed at almost equal distance from two ammonium groups from the adjacent molecules (3.131 and 3.169 Å, respectively, Fig. 9a); at the same time, hydrogen atoms C–H9 and C–H8 from two surrounding molecules formed close contacts with the same chloride ion (2.88 and 2.918 Å, respectively, Fig. 10.). Torsional angles C7C8N1C10 were identical in both enantiomers present in the elementary cell (165.77o).
Compound 2
Compound 2 stereoisomers occurred pairwise forming hydrogen bonds with chloride ions (Fig. 11). Torsional angles C7C8N1C10 are identical in both enantiomers (69.25°). Distances between N-HA, as well as N-HB, and chloride ion present between these molecules were 2.221 and 2.322Å, respectively.
a Two molecules forming half an elementary cell of compound 2 with short interatomic distances are shown. b Three molecules of compound 2 surrounding the chloride anion in the crystallographic structure. The dotted lines show short contacts of chloride ion with hydrogen atoms from three adjacent molecules
Crystallographic structures of both compounds can be characterized by the distance between the chloride ion and the surrounding molecules of cathinone cation. In the case of compound 1, the chloride ion was surrounded by four different cationic species (Fig. 10), whereas in compound 2, this ion was surrounded by three adjacent cations (Fig. 11b). This finding is corroborated by short distances between the chloride ion and various fragments of surrounding molecules (<3Å).
Conclusions
In this paper, we report spectroscopic (IR, NMR and MS) and crystallographic characteristics of two designer drugs: ethcathinone and 4-chloroethcathinone (4-CEC), both present on the illicit drug market either as pure substance or mixed with other species (to be confirmed chromatographically as well as by using NMR and MS). The examined samples of these two compounds proved, indeed, to be either very pure (compound 1) or mixed with other substances (compound 2). NMR and MS spectra can be used to differentiate between both compounds. Crystallographic studies confirmed the occurrence of both compounds as racemic mixtures which would rather be expected considering the possible method of synthesizing these compounds.
References
European Monitoring Centre for Drugs and Drug Addiction (2015) European drug report. http://www.emcdda.europa.eu/attachements.cfm/att_239505_EN_TDAT15001ENN.pdf. Accessed June 2015
Zawilska J, Wojcieszak J (2013) Designer cathinones—an emerging class of novel psychoactive substances. Forensic Sci Int 231:42–53
Valente MJ, Geudes de Pinho P, Carvalho F, Carvalho M (2014) Khat and synthetic cathinones: a review. Arch Toxicol 88:15–45
Prosser JM, Nelson LS (2012) The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol 8:33–42
http://gis.gov.pl/images/%C5%9Brodki_zast%C4%99pcze/%C5%9Bz/gis_raport_2013-2014.pdf. Accessed June 2015
Adamowicz P, Geroń J, Gil D, Lechowicz W, Skulska A, Tokarczyk B (2016) The prevalence of new psychoactive substances in biological material—a three-year review of casework in Poland. Drug Test Anal 8:63–70
Yu H, Rothman RB, Dersch CM, Partilla JS, Rice KC (2000) Uptake and release effects of diethylpropion and its metabolites and biogenic amine transporters. Bioorg Med Chem 8:2689–2692
Rosen E, Free SM, Heil GC (1971) Reduced side effects following administration of a new anorectic agent, 4′-chloro-2-(ethylamino)propiophenone, in timed-release form. J Pharm Sci 60:1900–1903
Dal Cason TA (2007) Synthesis and identification of N, N-dimethylcathinone hydrochloride. Microgram J 5:3–12
National Forensic Laboratory, Slovenia (2016) Analytical report: 4-CEC. http://www.policija.si/apps/nfl_response_web/0_Analytical_Reports_final/4-CEC-ID-1501-16-report_final.pdf_. Accessed Feb 2016
Nycz JE, Małecki G, Zawiązalec M, Paździorek T (2011) X-ray structures and computational studies of several cathinones. J Mol Struct 1002:10–18
Power JD, McDermott SD, Talbot B, O’Brien JE, Kavanagh P (2012) The analysis of amphetamine-like cathinone derivatives using positive electrosprey ionization with in-source collision-induced dissociation. Rapid Commun Mass Spectrom 26:2601–2611
Fornal E (2013) Formation of odd-electron product ions in collision-induced fragmentation of electrospray-generated protonated cathinone derivatives: aryl α-primary amino ketones. Rapid Commun Mass Spectrom 27:1858–1866
Hyde JF, Browning E, Adams R (1928) Synthetic homologs of d, l-ephedrine. J Am Chem Soc 50:2287–2292
Kavanagh P, O’Brien JE, Fox J, O’Donnell C, Christie R, Power JD, McDermott SD (2012) The analysis of substituted cathinones. Part 3. Synthesis and characterization of 2,3-methylenedioxy substituted cathinones. Forensic Sci Int 216:19–28
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A 64:112–122
Camilleri A, Johnson MR, Brennan M, Davis S, Caldicott DGE (2010) Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, α- phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int 197:59–66
Awad T, Clarke CR, DeRuiter J (2006) Chromatographic and mass spectral studies on methoxymethcathinones related to 3,4-methylenedioxymethamphetamine. J Chromatogr Sci 44:155–161
Majchrzak M, Rojkiewicz M, Celiński R, Kuś P, Sajewicz M (2016) Identification and characterization of new designer drug 4-fluoro-PV9 and α-PHP in the seized materials. Forensic Toxicol 34:115–124
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or other relations that could lead to a conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kuś, P., Kusz, J., Książek, M. et al. Spectroscopic characterization and crystal structures of two cathinone derivatives: N-ethyl-2-amino-1-phenylpropan-1-one (ethcathinone) hydrochloride and N-ethyl-2-amino-1-(4-chlorophenyl)propan-1-one (4-CEC) hydrochloride. Forensic Toxicol 35, 114–124 (2017). https://doi.org/10.1007/s11419-016-0345-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-016-0345-6