Abstract
Purpose
The objective of this research was to apply the same immobilization (stabilization/solidification) clay-based treatments to sediment contaminated with different metals (Pb, Cd, Ni, Zn, Cu, Cr) with different distributions and availabilities in sediment. We also examined the possibility of using clay as an immobilization agent without the application of thermal treatment, in order to reduce the economic cost of this expensive remediation procedure.
Materials and methods
Clay from a canal in Vojvodina, Republic of Serbia, was used as the immobilization agent in a stabilization/solidification treatment to remediate metal-contaminated sediment. Semi-dynamic and toxicity characteristic leaching tests were conducted to assess the effectiveness of the nonthermal and thermal immobilization treatments with clay, and the long-term leaching behavior of these metals was determined using the following parameters: cumulative percentage of metals leached; diffusion coefficients; leachability indices; and toxicity characteristic leaching test concentration.
Results and discussion
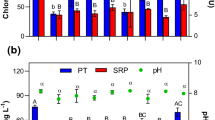
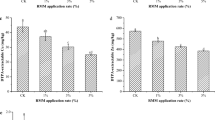
Based on these parameters, both clay-based treatments were effective in immobilizing metals in the contaminated sediment. Results suggest that both heating temperature and clay proportion in the sediment–clay mixture impact the degree of metal immobilization.
Conclusions
Clay-based products are potentially good immobilization materials for metal-contaminated sediments, with the distribution of metals in the original sediment not influencing the efficacy of the treatments. Even without the thermal treatment, the metals were effectively immobilized. The leaching of metals was largely inside the regulatory limits and the treated samples can be regarded as nonhazardous materials. This justifies the choice of not applying the more expensive thermal treatment during remediation, especially when treating sediments containing a mixture of pollutants.

Similar content being viewed by others
References
ANS (American National Standard) (1986) ANSI/ANS 16.1. American National Standard for the Measurement of the Leachibility of Solidified Low-Level Radioactive Wastes by Short-Term Tests Procedures. American National Standards Institute, New York
Antoniadis V, Tsadilas CD, Ashworth DJ (2007) Monometal and competitive adsorption of heavy metals by sewage sludge-amended soil. Chemosphere 68:489–494
Arain MB, Kazi TG, Jamali MK, Afridi HI, Jalbani N, Sarfraz RA (2008) Time saving modified BCR sequential extraction procedure for the fraction of Cd, Cr, Cu, Ni, Pb and Zn in sediment samples of polluted lake. J Hazard Mater 160:235–239
ASTM (American Society for Testing and Materials) (1993) Annual book of ASTM standards. Soil and rock; Building stones vol. 4.08. Philadelphia, PA, USA
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interf Sci 140:114–131
CCME (Canadian Council of Ministers of the Environment) (1995) Protocol for the derivation of Canadian sediment quality guidelines for the protection of aquatic life. CCME EPC-98E. Prepared by Environment Canada, Guideline Division, Technical Secretariat of the CCME Task Group on Water Quality Guidelines, Ottawa, Canada
Chiang KY, Chien KL, Hwang SW (2007) Study on the characteristics of building bricks produced from reservoir sediment. J Hazard Mater 159:499–504
Covelo EF, Vega FA, Andrade ML (2007) Competitive sorption and desorption of heavy metals by individual soil components. J Hazard Mater 140:308–315
Ciszewski D, Kubsik U, Kwaterezak UA (2012) Long-term dispersal of heavy metals in a catchment affected by historic lead and zinc mining. J Soils Sediments 12:1445–1462
Dermatas D, Moon DH, Menounou XM, Hires R (2004) An evaluation of arsenic release from monolithic solids using a modified semi-dynamic leaching test. J Hazard Mater B116:25–38
Environment Canada (1991) Proposed evaluation protocol for cement-based solidified wastes. Environmental Protection Series, Report No. EPS 3/HA/9, Environment Canada, Ottawa, Canada
Espinosa DCR, Tenorio JAS (2001) Thermal behavior of chromium electroplating sludge. Waste Manage 21:405–410
Fytianos K, Lourantou A (2004) Speciation of elements in sediment samples collected at lakes Volvi and Koronia, N. Greece. Environ Int 30:11–17
Gaskova O, Bukaty M (2008) Sorption of different cations onto clay minerals: modelling approach with ion exchange and surface complexation. Phys Chem Earth 33:1050–1055
Guevara-Riba A, Sahuquillo A, Rubio R, Rauret G (2004) Assessment of metal mobility in dredged harbour sediments from Barcelona, Spain. Sci Total Environ 321:241–255
Gupta SS, Bhattacharyya KG (2005) Interaction of metal ions with clays. I. A case study with Pb(II). Appl Clay Sci 30:199–208
ISO (International Organization for Standardization) (1995) ISO 11466: soil quality. Extraction of trace elements soluble in aqua regia, by International Organization for Standardization, 190/SC 3/WG 1, Geneva, Switzerland
Jain CK (2004) Metal fractionation study on bed sediments of River Yamuna, India. Water Res 38:569–578
Jamali MK, Kazi TG, Arain MB, Afridi HI, Jalbani N, Kandhro GA (2009) Speciation of heavy metals in untreated sewage sludge by using microwave assisted sequential extraction procedure. J Hazard Mater 163:1157–1164
Jiang MQ, Jin XY, Lu XQ, Chen ZL (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252:33–39
Kelderman P, Drossaert WME (1999) Characterization of polluted sediments in the city canals of Delft, the Netherlands. Proceedings CATS 4 (Characterization and Treatment of Sediments), Antwerp, Belgium; pp 133–142
Lafhaj Z, Samara M, Agostini F, Boucard L, Skoczylas F, Depelsenaire G (2008) Polluted river sediments from the North region of France: treatment with Novosol process and valorization in clay bricks. Constr Build Mater 22:755–762
Louriño-Cabana B, Lesven L, Charriau A, Billon G, Ouddane B, Boughriet A (2011) Potential risks of metal toxicity in contaminated sediments of Deűle river in Northern France. J Hazard Mater 186:2129–2137
MHSPEDGEP (Ministry of Housing, Spatial Planning and Environment Directorate-General for Environmental Protection) (2000) Circular on target values and intervention values for soil remediation, Netherlands Government Gazette 39, Amsterdam, The Netherlands
Moon DH, Dermatas D (2006) An evaluation of lead leachability from stabilized/solidified soils under mofied semi-dynamic leaching conditions. Engin Geol 85:67–74
Moon DH, Dermatas D (2007) Arsenic and lead release from fly ash stabilized/solidified soils under modified semi-dynamic leaching conditions. J Hazard Mater 141:388–394
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy remediation of dredged sediments. J Hazard Mater 85:145–163
Nathwani JS, Phillips CR (1980) Leachability of Ra-226 from uranium mill tailings consolidated with naturally occurring materials and/or cement: analysis based on mass transport equation. Water Air Soil Pollut 14:389–402
NEN (Netherlands Standardization Institute) (1990a) Determination of copper content by atomic absorption spectrometry (flame technique), Netherlands norm, NEN 5758, Netherlands Standardization Institute, Delft, the Netherlands
NEN (1990b) Determination of zinc content by atomic absorption spectrometry (flame technique), Netherlands norm, NEN 5759, Netherlands Standardization Institute, Delft, the Netherlands
NEN (1990c) Determination of lead content by atomic absorption 775 spectrometry (flame technique), Netherlands norm, NEN 5761, Netherlands Standardization Institute, Delft, the Netherlands
NEN (1990d) Determination of cadmium content by atomic absorption spectrometry (flame technique), Netherlands norm, NEN 5762, Netherlands Standardization Institute, Delft, the Netherlands
NEN (1991a) Determination of nickel content by atomic absorption spectrometry (flame technique), Netherlands norm, NEN 5765, Netherlands Standardization Institute, Delft, the Netherlands
NEN (1991b) Determination of chromium content by atomic absorption spectrometry (flame technique), Netherlands norm, NEN 5767, Netherlands Standardization Institute, Delft, the Netherlands
NEN (1994) Determination of organic matter content in soil as loss-on-ignition, Netherlands norm, NEN 5754, Netherlands Standardization Institute, Delft, the Netherlands
Rengaraj S, Yeon KH, Kang SY, Lee JU, Kim KW, Moon SH (2002) Studies on adsorptive removal of Co(II), Cr(III) and Ni(II) by IRN77 cation-exchange resin. J Hazard Mater B92:185–198
Shukla A, Zhang YH, Dubey P, Margrave JL, Shukla SS (2002) The role of sawdust in the removal of unwanted materials from water. J Hazard Mater 95:137–152
Sipos P, Németh T, Kovács KV, Mohai I (2008) Sorption of copper, zinc and lead on soil mineral phases. Chemosphere 73:461–469
Singh IB, Chaturvedi K, Morchhale RK, Yegneswaran AH (2007) Thermal treatment of toxic metals of industrial hazardous wastes with fly ash and clay. J Hazard Mater 141:215–222
Tuzen M, Melek E, Soylak M (2006) Celtek clay as sorbent for separation–preconcentration of metal ions from environmental samples. J Hazard Mater B 136:597–603
USEPA (2002) Toxicity characteristic leaching procedure, Method 1311. Available at: www.EPA.gov/SW-846/1311.pdf. Accessed 16 Apr 2012
Wang KS, Sun CJ, Liu CY (2001) Effects of the type of sintering atmosphere on the chromium leachability of thermal-treated municipal solid waste incinerator fly ash. Waste Manage 21:85–91
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater B 80:33–42
Zhu J, Pigna M, Cozzolino V, Caporale AG, Violante A (2010) Competitive sorption of copper(II), chromium(III) and lead(II) on ferrihydrite and two organomineral complexes. Geoderma 159:409–416
Acknowledgments
The authors acknowledge the financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant nos. III43005 and TR37004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Trudy J. Estes
Rights and permissions
About this article
Cite this article
Krcmar, D., Dalmacija, M., Dalmacija, B. et al. Evaluating the necessity for thermal treatment in clay-based metal immobilization techniques as an environmentally acceptable sediment remediation process. J Soils Sediments 13, 1318–1326 (2013). https://doi.org/10.1007/s11368-013-0722-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0722-2