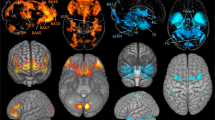
Abstract
Neuroanatomical sex differences estimated in neuroimaging studies are confounded by total intracranial volume (TIV) as a major biological factor. Employing a matching approach widely used for causal modeling, we disentangled the effect of TIV from sex to study sex-differentiated brain aging trajectories, their relation to functional networks and cytoarchitectonic classes, brain allometry, and cognition. Using data from the UK Biobank, we created subsamples that removed, maintained, or exaggerated the TIV differences in the original sample. We compared regional and vertex-level sex estimates across subsamples. The overall sex-related differences diminished in head size–matched subsamples, suggesting that most of the observed variability results from TIV differences. Furthermore, bidirectional sex differences in brain neuroanatomy emerged that were previously masked by the effect of TIV. Allometry remained fairly consistent across lifespan and was not sex-differentiated. Finally, the matching process changed the direction of the estimated sex differences in “verbal and numerical reasoning” and “working memory”, suggesting that behavioral sex difference investigations can benefit from additional biological analysis to uncover the underlying factors contributing to cognition. Taken together, we provide new evidence disentangling sex differences from TIV as a relevant biological confound.




Similar content being viewed by others
Data Availability
The data used in this work was obtained from the UKBB under approved application 45551. UKBB data can be accessed through their access management system (https://www.ukbiobank.ac.uk/enable-your-research/apply-foraccess).
References
Hou Y, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):10. https://doi.org/10.1038/s41582-019-0244-7.
Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol Mech Dis. 2008;3(1):41–66. https://doi.org/10.1146/annurev.pathmechdis.2.010506.092044.
Bethlehem RAI, et al. Brain charts for the human lifespan. Nature. 2022;604(7906):525–33. https://doi.org/10.1038/s41586-022-04554-y.
Narvacan K, Treit S, Camicioli R, Martin W, Beaulieu C. Evolution of deep gray matter volume across the human lifespan. Hum Brain Mapp. 2017;38(8):3771–90. https://doi.org/10.1002/hbm.23604.
Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging. 2011;32(5):907–15. https://doi.org/10.1016/j.neurobiolaging.2009.05.003.
Frangou S, et al. Cortical thickness across the lifespan: data from 17,075 healthy individuals aged 3–90 years. Hum Brain Mapp. 2021;43(1):431–51. https://doi.org/10.1002/hbm.25364.
Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52(4):1215–23. https://doi.org/10.1016/j.neuroimage.2010.04.258.
Zeighami Y, Evans AC. Association vs. prediction: the impact of cortical surface smoothing and parcellation on brain age. Front Big Data. 2021;4. https://doi.org/10.3389/fdata.2021.637724.
Schilling KG, et al. Aging and white matter microstructure and macrostructure: a longitudinal multi-site diffusion MRI study of 1218 participants. Brain Struct Funct. 2022;227(6):2111–25. https://doi.org/10.1007/s00429-022-02503-z.
Garnier-Crussard A, et al. White matter hyperintensities across the adult lifespan: relation to age, Aβ load, and cognition. Alzheimers Res Ther. 2020;12(1):127. https://doi.org/10.1186/s13195-020-00669-4.
Phyo AZZ, Fransquet PD, Wrigglesworth J, Woods RL, Espinoza SE, Ryan J. Sex differences in biological aging and the association with clinical measures in older adults. GeroScience. 2023. https://doi.org/10.1007/s11357-023-00941-z.
Ruigrok ANV, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. https://doi.org/10.1016/j.neubiorev.2013.12.004.
Wierenga LM, et al. Greater male than female variability in regional brain structure across the lifespan. Hum Brain Mapp. 2020;43(1):470–99. https://doi.org/10.1002/hbm.25204.
Vinke EJ, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging. 2018;71:32–40. https://doi.org/10.1016/j.neurobiolaging.2018.07.001.
Ferretti MT, et al. Sex differences in Alzheimer disease — the gateway to precision medicine. Nat Rev Neurol. 2018;14(8):8. https://doi.org/10.1038/s41582-018-0032-9.
Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15(5):451–2. https://doi.org/10.1016/S1474-4422(16)00067-3.
Meoni S, Macerollo A, Moro E. Sex differences in movement disorders. Nat Rev Neurol. 2020;16(2):2. https://doi.org/10.1038/s41582-019-0294-x.
Eliot L, Ahmed A, Khan H, Patel J. Dump the ‘dimorphism’: comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci Biobehav Rev. 2021;125:667–97. https://doi.org/10.1016/j.neubiorev.2021.02.026.
Ritchie SJ, et al. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex. 2018;28(8):2959–75. https://doi.org/10.1093/cercor/bhy109.
Farias ST, et al. Maximal brain size remains an important predictor of cognition in old age, independent of current brain pathology. Neurobiol Aging. 2012;33(8):1758–68. https://doi.org/10.1016/j.neurobiolaging.2011.03.017.
Liu D, Johnson HJ, Long JD, Magnotta VA, Paulsen JS. The power-proportion method for intracranial volume correction in volumetric imaging analysis. Front Neurosci. 2014;8:97423. https://doi.org/10.3389/fnins.2014.00356.
Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50(2):121–39. https://doi.org/10.1016/0925-4927(93)90016-b.
Sanchis-Segura C, et al. Sex differences in gray matter volume: how many and how large are they really? Biol Sex Differ. 2019;10(1):32. https://doi.org/10.1186/s13293-019-0245-7.
Sanchis-Segura C, Ibañez-Gual MV, Aguirre N, Cruz-Gómez ÁJ, Forn C. Effects of different intracranial volume correction methods on univariate sex differences in grey matter volume and multivariate sex prediction. Sci Rep. 2020;10(1):1. https://doi.org/10.1038/s41598-020-69361-9.
Williams CM, Peyre H, Toro R, Ramus F. Neuroanatomical norms in the UK Biobank: the impact of allometric scaling, sex, and age. Hum Brain Mapp. 2021;42(14):4623–42. https://doi.org/10.1002/hbm.25572.
Reardon PK, et al. Normative brain size variation and brain shape diversity in humans. Science. 2018;360(6394):1222–7. https://doi.org/10.1126/science.aar2578.
Im K, Lee J-M, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18(9):2181–91. https://doi.org/10.1093/cercor/bhm244.
Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 2009;29(45):14265–70. https://doi.org/10.1523/JNEUROSCI.2261-09.2009.
Luders E, Toga AW, Thompson PM. Why size matters: differences in brain volume account for apparent sex differences in callosal anatomy: the sexual dimorphism of the corpus callosum. Neuroimage. 2014;84:820–4. https://doi.org/10.1016/j.neuroimage.2013.09.040.
Sowell ER, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550–60. https://doi.org/10.1093/cercor/bhl066.
Kurth F, Thompson PM, Luders E. Investigating the differential contributions of sex and brain size to gray matter asymmetry. Cortex. 2018;99:235–42. https://doi.org/10.1016/j.cortex.2017.11.017.
Voevodskaya O, et al. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front Aging Neurosci. 2014;6. https://doi.org/10.3389/fnagi.2014.00264.
Wang L, Shen H, Tang F, Zang Y, Hu D. Combined structural and resting-state functional MRI analysis of sexual dimorphism in the young adult human brain: an MVPA approach. Neuroimage. 2012;61(4):931–40. https://doi.org/10.1016/j.neuroimage.2012.03.080.
Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 2015;12(3):e1001779. https://doi.org/10.1371/journal.pmed.1001779.
Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–81. https://doi.org/10.1016/j.neuroimage.2012.01.021.
Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Klasson N, Olsson E, Eckerström C, Malmgren H, Wallin A. Estimated intracranial volume from FreeSurfer is biased by total brain volume. Eur Radiol Exp. 2018;2(1):24. https://doi.org/10.1186/s41747-018-0055-4.
Dadar M, Fonov VS, Collins DL. A comparison of publicly available linear MRI stereotaxic registration techniques. Neuroimage. 2018;174:191–200. https://doi.org/10.1016/j.neuroimage.2018.03.025.
Dadar M, et al. Cerebral atrophy in amyotrophic lateral sclerosis parallels the pathological distribution of TDP43. Brain Commun. 2020;2(2):fcaa061. https://doi.org/10.1093/braincomms/fcaa061.
Dadar M, Narayanan S, Arnold DL, Collins DL, Maranzano J. Conversion of diffusely abnormal white matter to focal lesions is linked to progression in secondary progressive multiple sclerosis. Mult Scler J. 2021;27(2):208–19. https://doi.org/10.1177/1352458520912172.
Dadar M, Manera AL, Ducharme S, Collins DL. White matter hyperintensities are associated with grey matter atrophy and cognitive decline in Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging. 2022;111:54–63. https://doi.org/10.1016/j.neurobiolaging.2021.11.007.
Manera AL, et al. MRI data-driven algorithm for the diagnosis of behavioural variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2021;92(6):608–16. https://doi.org/10.1136/jnnp-2020-324106.
Misquitta K, Dadar M, Louis Collins D, Tartaglia MC. White matter hyperintensities and neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease. NeuroImage Clin. 2020;28:102367. https://doi.org/10.1016/j.nicl.2020.102367.
Zeighami Y, Fereshtehnejad S-M, Dadar M, Collins DL, Postuma RB, Dagher A. Assessment of a prognostic MRI biomarker in early de novo Parkinson’s disease. NeuroImage Clin. 2019;24:101986. https://doi.org/10.1016/j.nicl.2019.101986.
Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging. 2008;27(4):425–41. https://doi.org/10.1109/TMI.2007.906087.
Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. https://doi.org/10.1109/42.668698.
Alfaro-Almagro F, et al. Confound modelling in UK Biobank brain imaging. Neuroimage. 2021;224:117002. https://doi.org/10.1016/j.neuroimage.2020.117002.
Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65. https://doi.org/10.1152/jn.00338.2011.
Scholtens LH, de Reus MA, de Lange SC, Schmidt R, van den Heuvel MP. An MRI Von Economo – Koskinas atlas. Neuroimage. 2018;170:249–56. https://doi.org/10.1016/j.neuroimage.2016.12.069.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300.
Moqadam R, Dadar M, Zeighami Y. Investigating the impact of motion in the scanner on brain age predictions. Imaging Neurosci. 2024;2:1–21. https://doi.org/10.1162/imag_a_00079.
Potvin O, Mouiha A, Dieumegarde L, Duchesne S. Normative data for subcortical regional volumes over the lifetime of the adult human brain. Neuroimage. 2016;137:9–20. https://doi.org/10.1016/j.neuroimage.2016.05.016.
Liu S, Seidlitz J, Blumenthal JD, Clasen LS, Raznahan A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc Natl Acad Sci. 2020;117(31):18788–98. https://doi.org/10.1073/pnas.1919091117.
Jäncke L, Mérillat S, Liem F, Hänggi J. Brain size, sex, and the aging brain. Hum Brain Mapp. 2015;36(1):150–69. https://doi.org/10.1002/hbm.22619.
Sanchis-Segura C, Aguirre N, Cruz-Gómez ÁJ, Félix S, Forn C. Beyond ‘sex prediction’: estimating and interpreting multivariate sex differences and similarities in the brain. Neuroimage. 2022;257:119343. https://doi.org/10.1016/j.neuroimage.2022.119343.
Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth. 1909
Zilles K, Amunts K. Centenary of Brodmann’s map — conception and fate. Nat Rev Neurosci. 2010;11(2):2. https://doi.org/10.1038/nrn2776.
von Economo CF, Koskinas GN. Die cytoarchitektonik der hirnrinde des erwachsenen menschen. J Springer. 1925
Schaefer A, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28(9):3095–114. https://doi.org/10.1093/cercor/bhx179.
Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):7615. https://doi.org/10.1038/nature18933.
Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. PLoS ONE. 2020;15(4):e0231627. https://doi.org/10.1371/journal.pone.0231627.
Littlejohns TJ, et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat Commun. 2020;11(1):1. https://doi.org/10.1038/s41467-020-15948-9.
Acknowledgements
This research was conducted using the UKBB Resource under approved application 45551. We thank the UKBB participants and team for their work in collecting, processing, and disseminating these data for analysis. The authors also acknowledge use of Compute Canada (https://alliancecan.ca/en) resources for performing the image processing analyses in the presented work.
Funding
This research was undertaken thanks in part to funding from the Canada First Research Excellence Fund and Fonds de recherche du Québec, awarded to the Healthy Brains, Healthy Lives (HBHL) initiative at McGill University. MD also reports receiving research funding from the Canadian Institutes of Health Research (CIHR), and Fonds de Recherche du Québec-Santé (FRQS). YZ reports receiving research funding from the FRQS Chercheurs boursiers en Intelligence artificielle, as well as Natural Sciences and Engineering Research (NSERC) discovery grant.
Author information
Authors and Affiliations
Contributions
A.B.R.: conceptualization, formal analysis, interpretation of findings, methodology, visualization, writing—original draft, writing—review and editing. R.M.: formal analysis, review and editing Y.I.M.: conceptualization, review and editing. M.C.: conceptualization, review and editing. M.D.: conceptualization, formal analysis, interpretation of findings, methodology, visualization, writing—original draft, writing—review and editing. Y.Z.: conceptualization, formal analysis, interpretation of findings, methodology, visualization, writing—original draft, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Brzezinski-Rittner, A., Moqadam, R., Iturria-Medina, Y. et al. Disentangling the effect of sex from brain size on brain organization and cognitive functioning. GeroScience 47, 247–262 (2025). https://doi.org/10.1007/s11357-024-01486-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-024-01486-5