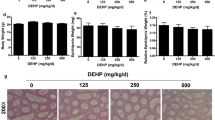
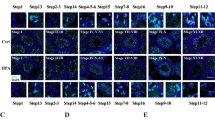
Abstract
Di-(2-ethylhexyl) phthalate (DEHP), a prevalent plasticizer, is known to have endocrine-disrupting effects on males and cause reproductive toxicity. There were causal effects of DEHP on testosterone levels in the real world by Mendelian randomization analysis. Exposure to DEHP during the preadult stage might lead to premature testicular senescence, but the mechanisms responsible for this have yet to be determined. In this study, we administered DEHP (300 mg/kg/day) to male C57BL/6 mice from postnatal days 21 to 49. The mice were kept for 6 months without DEHP. RNA sequencing was conducted on testicular tissue at PNM6. The results indicated that DEHP hindered testicular development, lowered serum testosterone levels in male mice, and induced premature testicular senescence. TM3 Leydig cells were exposed to 300 μM of mono(2-ethylhexyl) phthalate (MEHP), the bioactive metabolite of DEHP, for 72 h. The results also found that DEHP/MEHP induced senescence in vivo and in vitro. The mitochondrial respiratory chain was disrupted in Leydig cells. The expression and stability of STAT5B were elevated by MEHP treatment in TM3 cells. Furthermore, p-ERK1/2 was significantly decreased by STAT5B, and mitochondria-STAT3 (p-STAT3 ser727) was significantly decreased due to the decrease of p-ERK1/2. Additionally, the senescence level of TM3 cells was decreased and treated with 5 mM NAC for 1 h after MEHP treatment. In conclusion, these findings provided a novel mechanistic understanding of Leydig cells by disrupting the mitochondrial respiratory chain through STAT5B-mitoSTAT3.







Similar content being viewed by others
Data availability
The data supporting the results of this study are available from the corresponding author upon any reasonable request.
Abbreviations
- DEHP :
-
Di-(2-ethylhexyl) phthalate
- MEHP :
-
Mono(2-ethylhexyl) phthalate
- TD :
-
Testosterone deficiency
- ROS :
-
Reactive oxygen species
- RNS :
-
Reactive nitrogen species
- MR :
-
Mendelian randomization
- IV :
-
Instrumental variable
- SNPs :
-
Single nucleotide polymorphisms
- BAT :
-
Bioavailable testosterone
- SHBG :
-
Sex hormone-binding globulin
- DMRs :
-
Differentially methylated regions
- LD :
-
Linkage disequilibrium
- PND :
-
Postnatal day
- PNM :
-
Postnatal month
- DEGs :
-
Differentially expressed genes
- AGD :
-
Anogenital distance
- IHC :
-
Immunohistochemical
- IF :
-
Immunofluorescence
- DEGs :
-
Differentially expressed genes
- FDR :
-
False discovery rate
- GO :
-
Gene Ontology
- GSEA :
-
Gene set enrichment analysis
- CS :
-
Cell senescence
- OXPHOS :
-
Oxidative phosphorylation
- ssGSEA :
-
Single-sample gene set enrichment analysis
- SAHF :
-
Senescence-associated heterochromatin foci
- OCR :
-
Oxygen consumption rate
- EDI :
-
Estimated daily intake
- COIP :
-
Co-immunoprecipitation
- CESTA :
-
Cellular thermal shift assay
- NAC :
-
N-acetyl-cysteine
References
Salter CA, Mulhall JP. Guideline of guidelines: testosterone therapy for testosterone deficiency. BJU Int. 2019;124:722–9. https://doi.org/10.1111/bju.14899.
Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R25-45. https://doi.org/10.1530/JOE-12-0455.
Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–32. https://doi.org/10.1016/j.juro.2018.03.115.
Kloner RA, Carson C, Dobs A, Kopecky S, Mohler ER. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545–57. https://doi.org/10.1016/j.jacc.2015.12.005.
Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15:1260–71. https://doi.org/10.1016/j.jsxm.2018.06.012.
Martínez-Razo LD, Martínez-Ibarra A, Vázquez-Martínez ER, Cerbón M. The impact of di-(2-ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in placental development, function, and pathophysiology. Environ Int. 2021;146:106228. https://doi.org/10.1016/j.envint.2020.106228.
Khasin LG, Della Rosa J, Petersen N, Moeller J, Kriegsfeld LJ, Lishko PV. The impact of di-2-ethylhexyl phthalate on sperm fertility. Front Cell Dev Biol. 2020;8:426. https://doi.org/10.3389/fcell.2020.00426.
Burdorf A, Brand T, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EAP. The effects of work-related maternal risk factors on time to pregnancy, preterm birth and birth weight: the Generation R Study. Occup Environ Med. 2011;68:197–204. https://doi.org/10.1136/oem.2009.046516.
Wu Y, Wang J, Zhao T, Chen J, Kang L, Wei Y, et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J Hazard Mater. 2022;426:127807. https://doi.org/10.1016/j.jhazmat.2021.127807.
Zhao T-X, Wang J-K, Shen L-J, Long C-L, Liu B, Wei Y, et al. Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ Pollut. 2020;259:113911. https://doi.org/10.1016/j.envpol.2020.113911.
Hong Y, Zhou Y, Shen L, Wei Y, Long C, Fu Y, et al. Exposure to DEHP induces testis toxicity and injury through the ROS/mTOR/NLRP3 signaling pathway in immature rats. Ecotoxicol Environ Saf. 2021;227:112889. https://doi.org/10.1016/j.ecoenv.2021.112889.
Santiago J, Silva JV, Alves MG, Oliveira PF, Fardilha M. Testicular aging: an overview of ultrastructural, cellular, and molecular alterations. J Gerontol A Biol Sci Med Sci. 2019;74:860–71. https://doi.org/10.1093/gerona/gly082.
Nelson JF, Latham KR, Finch CE. Plasma testosterone levels in C57BL/6J male mice: effects of age and disease. Acta Endocrinol (Copenh). 1975;80:744–52. https://doi.org/10.1530/acta.0.0800744.
Sokanovic SJ, Baburski AZ, Janjic MM, Stojkov NJ, Bjelic MM, Lalosevic D, et al. The opposing roles of nitric oxide and cGMP in the age-associated decline in rat testicular steroidogenesis. Endocrinology. 2013;154:3914–24. https://doi.org/10.1210/en.2013-1307.
Sokanovic SJ, Janjic MM, Stojkov NJ, Baburski AZ, Bjelic MM, Andric SA, et al. Age related changes of cAMP and MAPK signaling in Leydig cells of Wistar rats. Exp Gerontol. 2014;58:19–29. https://doi.org/10.1016/j.exger.2014.07.004.
Barakat R, Lin P-CP, Rattan S, Brehm E, Canisso IF, Abosalum ME, et al. Prenatal exposure to DEHP induces premature reproductive senescence in male mice. Toxicol Sci. 2017;156:96–108. https://doi.org/10.1093/toxsci/kfw248.
Qigen X, Haiming C, Kai X, Yong G, Chunhua D. Prenatal DEHP exposure induces premature testicular aging by promoting Leydig cell senescence through the MAPK signaling pathways. Adv Biol (Weinh). 2023;7:e2300130. https://doi.org/10.1002/adbi.202300130.
Minutoli L, Puzzolo D, Rinaldi M, Irrera N, Marini H, Arcoraci V, et al. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev. 2016;2016:2183026. https://doi.org/10.1155/2016/2183026.
Wei Y, Zhou Y, Long C, Wu H, Hong Y, Fu Y, et al. Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-Mediated imbalance of mTORC1 and mTORC2. Environ Pollut. 2021;289:117904. https://doi.org/10.1016/j.envpol.2021.117904.
Salomon TB, Hackenhaar FS, Almeida AC, Schüller AK, Gil Alabarse PV, Ehrenbrink G, et al. Oxidative stress in testis of animals during aging with and without reproductive activity. Exp Gerontol. 2013;48:940–6. https://doi.org/10.1016/j.exger.2013.06.010.
Li Y, Chen H, Liao J, Chen K, Javed MT, Qiao N, et al. Long-term copper exposure promotes apoptosis and autophagy by inducing oxidative stress in pig testis. Environ Sci Pollut Res Int. 2021;28:55140–53. https://doi.org/10.1007/s11356-021-14853-y.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26:252–8. https://doi.org/10.1038/s41591-020-0751-5.
Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. https://doi.org/10.1210/jcem.84.10.6079.
Ho CKM, Stoddart M, Walton M, Anderson RA, Beckett GJ. Calculated free testosterone in men: comparison of four equations and with free androgen index. Ann Clin Biochem. 2006;43:389–97. https://doi.org/10.1258/000456306778520115.
Green R, Allen LH, Bjørke-Monsen A-L, Brito A, Guéant J-L, Miller JW, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040. https://doi.org/10.1038/nrdp.2017.40.
Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–8. https://doi.org/10.1016/j.mad.2005.10.004.
Stajnko A, Runkel AA, Kosjek T, Snoj Tratnik J, Mazej D, Falnoga I, et al. Assessment of susceptibility to phthalate and DINCH exposure through CYP and UGT single nucleotide polymorphisms. Environ Int. 2022;159:107046. https://doi.org/10.1016/j.envint.2021.107046.
Wu H, Estill MS, Shershebnev A, Suvorov A, Krawetz SA, Whitcomb BW, et al. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: a cross-sectional study. Hum Reprod. 2017;32:2159–69. https://doi.org/10.1093/humrep/dex283.
Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol. 2004;78:123–30. https://doi.org/10.1007/s00204-003-0522-3.
Kessler W, Numtip W, Völkel W, Seckin E, Csanády GA, Pütz C, et al. Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol Appl Pharmacol. 2012;264:284–91. https://doi.org/10.1016/j.taap.2012.08.009.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. https://doi.org/10.1093/nar/28.1.27.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2:100141. https://doi.org/10.1016/j.xinn.2021.100141.
Avelar RA, Ortega JG, Tacutu R, Tyler EJ, Bennett D, Binetti P, et al. A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol. 2020;21:91. https://doi.org/10.1186/s13059-020-01990-9.
Chatsirisupachai K, Palmer D, Ferreira S, de Magalhães JP. A human tissue-specific transcriptomic analysis reveals a complex relationship between aging, cancer, and cellular senescence. Aging Cell. 2019;18:e13041. https://doi.org/10.1111/acel.13041.
Wang X, Ma L, Pei X, Wang H, Tang X, Pei J-F, et al. Comprehensive assessment of cellular senescence in the tumor microenvironment. Brief Bioinform. 2022;23:bbac118. https://doi.org/10.1093/bib/bbac118.
Saul D, Kosinsky RL, Atkinson EJ, Doolittle ML, Zhang X, LeBrasseur NK, et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun. 2022;13:4827. https://doi.org/10.1038/s41467-022-32552-1.
Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. https://doi.org/10.1186/1471-2105-14-7.
Rath S, Sharma R, Gupta R, Ast T, Chan C, Durham TJ, et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;49:D1541-7. https://doi.org/10.1093/nar/gkaa1011.
Guillot G, Rousset F. Dismantling the Mantel tests. Methods Ecol Evol. 2013;4:336–44. https://doi.org/10.1111/2041-210x.12018.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. https://doi.org/10.1038/nmeth.3337.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. https://doi.org/10.1073/pnas.0506580102.
Schubert M, Klinger B, Klünemann M, Sieber A, Uhlitz F, Sauer S, et al. Perturbation-response genes reveal signaling footprints in cancer gene expression. Nat Commun. 2018;9:20. https://doi.org/10.1038/s41467-017-02391-6.
Holland CH, Szalai B, Saez-Rodriguez J. Transfer of regulatory knowledge from human to mouse for functional genomics analysis. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194431. https://doi.org/10.1016/j.bbagrm.2019.194431.
Luo P, Feng X, Deng R, Wang F, Zhang Y, Li X, et al. An autofluorescence-based isolation of Leydig cells for testosterone deficiency treatment. Mol Cell Endocrinol. 2021;535:111389. https://doi.org/10.1016/j.mce.2021.111389.
Chandra T, Kirschner K, Thuret J-Y, Pope BD, Ryba T, Newman S, et al. Independence of repressive histone marks and chromatin compaction during senescent heterochromatic layer formation. Mol Cell. 2012;47:203–14. https://doi.org/10.1016/j.molcel.2012.06.010.
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. Cellular senescence: defining a path forward. Cell. 2019;179:813–27. https://doi.org/10.1016/j.cell.2019.10.005.
Letts JA, Fiedorczuk K, Sazanov LA. The architecture of respiratory supercomplexes. Nature. 2016;537:644–8. https://doi.org/10.1038/nature19774.
Salminen A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev. 2022;75:101573. https://doi.org/10.1016/j.arr.2022.101573.
Maceira A, Pecikoza I, Marcé RM, Borrull F. Multi-residue analysis of several high-production-volume chemicals present in the particulate matter from outdoor air. A preliminary human exposure estimation. Chemosphere. 2020;252:126514. https://doi.org/10.1016/j.chemosphere.2020.126514.
Wang J, Zhao T, Chen J, Kang L, Wei Y, Wu Y, et al. Multiple transcriptomic profiling: p53 signaling pathway is involved in DEHP-induced prepubertal testicular injury via promoting cell apoptosis and inhibiting cell proliferation of Leydig cells. J Hazard Mater. 2021;406:124316. https://doi.org/10.1016/j.jhazmat.2020.124316.
Xu J, Wang L, Zhang L, Zheng F, Wang F, Leng J, et al. Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity. Redox Biol. 2021;38:101776. https://doi.org/10.1016/j.redox.2020.101776.
Kato K, Silva MJ, Reidy JA, Hurtz D, Malek NA, Needham LL, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112:327–30. https://doi.org/10.1289/ehp.6663.
Silva MJ, Reidy JA, Preau JL, Samandar E, Needham LL, Calafat AM. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers. 2006;11:1–13. https://doi.org/10.1080/13547500500382868.
Sharma RP, Schuhmacher M, Kumar V. Development of a human physiologically based pharmacokinetic (PBPK) model for phthalate (DEHP) and its metabolites: a bottom up modeling approach. Toxicol Lett. 2018;296:152–62. https://doi.org/10.1016/j.toxlet.2018.06.1217.
Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–76. https://doi.org/10.1007/s00204-004-0642-4.
Zhang Q, Sun Y, Zhang Q, Hou J, Wang P, Kong X, et al. Phthalate exposure in Chinese homes and its association with household consumer products. Sci Total Environ. 2020;719:136965. https://doi.org/10.1016/j.scitotenv.2020.136965.
Chen H, Yang X, Gielen G, Mandal S, Xu S, Guo J, et al. Effect of biochars on the bioavailability of cadmium and di-(2-ethylhexyl) phthalate to Brassica chinensis L. in contaminated soils. Sci Total Environ. 2019;678:43–52. https://doi.org/10.1016/j.scitotenv.2019.04.417.
He M-J, Lu J-F, Wang J, Wei S-Q, Hageman KJ. Phthalate esters in biota, air and water in an agricultural area of western China, with emphasis on bioaccumulation and human exposure. Sci Total Environ. 2020;698:134264. https://doi.org/10.1016/j.scitotenv.2019.134264.
Malarvannan G, Onghena M, Verstraete S, van Puffelen E, Jacobs A, Vanhorebeek I, et al. Phthalate and alternative plasticizers in indwelling medical devices in pediatric intensive care units. J Hazard Mater. 2019;363:64–72. https://doi.org/10.1016/j.jhazmat.2018.09.087.
Tang Z, Chai M, Wang Y, Cheng J. Phthalates in preschool children’s clothing manufactured in seven Asian countries: occurrence, profiles and potential health risks. J Hazard Mater. 2020;387:121681. https://doi.org/10.1016/j.jhazmat.2019.121681.
Shen L, Tang X, Wei Y, Long C, Tan B, Wu S, et al. Vitamin E and vitamin C attenuate Di-(2-ethylhexyl) phthalate-induced blood-testis barrier disruption by p38 MAPK in immature SD rats. Reprod Toxicol. 2018;81:17–27. https://doi.org/10.1016/j.reprotox.2018.06.015.
Yi WEI, Xiang-Liang T, Yu Z, Bin L, Lian-Ju S, Chun-Lan L, et al. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis. 2018;5:263–74. https://doi.org/10.1016/j.gendis.2018.06.004.
Oudir M, Chader H, Bouzid B, Bendisari K, Latreche B, Boudalia S, et al. Male rat exposure to low dose of di(2-ethylhexyl) phthalate during pre-pubertal, pubertal and post-pubertal periods: impact on sperm count, gonad histology and testosterone secretion. Reprod Toxicol. 2018;75:33–9. https://doi.org/10.1016/j.reprotox.2017.11.004.
Perobelli JE. The male peripubertal phase as a developmental window for reproductive toxicology studies. Curr Pharm Des. 2014;20:5398–415. https://doi.org/10.2174/1381612820666140205150059.
Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci. 2016;150:97–108. https://doi.org/10.1093/toxsci/kfv317.
How CM, Yen P-L, Wei C-C, Li S-W, Liao VH-C. Early life exposure to di(2-ethylhexyl)phthalate causes age-related declines associated with insulin/IGF-1-like signaling pathway and SKN-1 in Caenorhabditis elegans. Environ Pollut. 2019;251:871–8. https://doi.org/10.1016/j.envpol.2019.04.141.
Pradhan A, Olsson P-E, Jass J. Di(2-ethylhexyl) phthalate and diethyl phthalate disrupt lipid metabolism, reduce fecundity and shortens lifespan of Caenorhabditis elegans. Chemosphere. 2018;190:375–82. https://doi.org/10.1016/j.chemosphere.2017.09.123.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. https://doi.org/10.1042/BJ20081386.
Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:3565127. https://doi.org/10.1155/2016/3565127.
van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–46. https://doi.org/10.1038/nature13193.
Traore K, More P, Adla A, Dogbey G, Papadopoulos V, Zirkin B. MEHP induces alteration of mitochondrial function and inhibition of steroid biosynthesis in MA-10 mouse tumor Leydig cells. Toxicology. 2021;463:152985. https://doi.org/10.1016/j.tox.2021.152985.
Tripathi A, Pandey V, Sahu AN, Singh A, Dubey PK. Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells. Toxicol Res (Camb). 2019;8:381–94. https://doi.org/10.1039/c8tx00263k.
Szczepanek K, Lesnefsky EJ, Larner AC. Multi-tasking: nuclear transcription factors with novel roles in the mitochondria. Trends Cell Biol. 2012;22:429–37. https://doi.org/10.1016/j.tcb.2012.05.001.
Wegrzyn J, Potla R, Chwae Y-J, Sepuri NBV, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7. https://doi.org/10.1126/science.1164551.
Kramer AH, Joos-Vandewalle J, Edkins AL, Frost CL, Prinsloo E. Real-time monitoring of 3T3-L1 preadipocyte differentiation using a commercially available electric cell-substrate impedance sensor system. Biochem Biophys Res Commun. 2014;443:1245–50. https://doi.org/10.1016/j.bbrc.2013.12.123.
Stephens JM, Morrison RF, Pilch PF. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J Biol Chem. 1996;271:10441–4. https://doi.org/10.1074/jbc.271.18.10441.
Tammineni P, Anugula C, Mohammed F, Anjaneyulu M, Larner AC, Sepuri NBV. The import of the transcription factor STAT3 into mitochondria depends on GRIM-19, a component of the electron transport chain. J Biol Chem. 2013;288:4723–32. https://doi.org/10.1074/jbc.M112.378984.
Liu C, Qian P, Yang L, Zhang L, Chen C, He M, et al. Pubertal exposure to di-(2-ethylhexyl)-phthalate inhibits G9a-mediated histone methylation during spermatogenesis in mice. Arch Toxicol. 2016;90:955–69. https://doi.org/10.1007/s00204-015-1529-2.
Acknowledgements
We are grateful to all the teachers (Ruiqing Han, Peking Union Medical College, and so on) for their help.
Author information
Authors and Affiliations
Contributions
HMC and QGX: conceptualization, investigation, visualization, writing — original draft.
HMC and JQC: investigation, data curation, visualization, software.
HMC: methodology, visualization, writing — revised draft.
PL: investigation, validation, methodology, visualization, writing — revised draft.
KX: investigation, data curation.
LM: investigation, validation.
MDC: conceptualization, supervision, investigation.
ZW: conceptualization, funding acquisition, project administration, writing — review and editing.
CHD: conceptualization, supervision, writing — review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Cao, H., Xie, Q., Luo, P. et al. Di-(2-ethylhexyl) phthalate exposure induces premature testicular senescence by disrupting mitochondrial respiratory chain through STAT5B-mitoSTAT3 in Leydig cell. GeroScience (2024). https://doi.org/10.1007/s11357-024-01119-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01119-x