Abstract
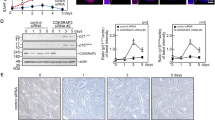
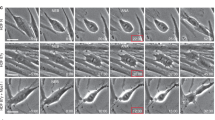
SMAD4 encodes a member of the SMAD family of proteins involved in the TGF-β signaling pathway. Potentially heritable, autosomal dominant, gain-of-function heterozygous variants of SMAD4 cause a rare developmental disorder, the Myhre syndrome, which is associated with a wide range of developmental and post-developmental phenotypes that we now characterize as a novel segmental progeroid syndrome. Whole-exome sequencing of a patient referred to our International Registry of Werner Syndrome revealed a heterozygous p.Arg496Cys variant of the SMAD4 gene. To investigate the role of SMAD4 mutations in accelerated senescence, we generated cellular models overexpressing either wild-type SMAD4 or mutant SMAD4-R496C in normal skin fibroblasts. We found that cells expressing the SMAD4-R496C mutant exhibited decreased proliferation and elevated expression of cellular senescence and inflammatory markers, including IL-6, IFNγ, and a TGF-β target gene, PAI-1. Here we show that transient exposure to TGF-β, an inflammatory cytokine, followed by chronic IFNγ stimulation, accelerated rates of senescence that were associated with increased DNA damage foci and SMAD4 expression. TGF-β, IFNγ, or combinations of both were not sufficient to reduce proliferation rates of fibroblasts. In contrast, TGF-β alone was able to induce preadipocyte senescence via induction of the mTOR protein. The mTOR inhibitor rapamycin mitigated TGF-β-induced expression of p21, p16, and DNA damage foci and improved replicative potential of preadipocytes, supporting the cell-specific response to this cytokine. These findings collectively suggest that persistent DNA damage and cross-talk between TGF-β/IFNγ pathways contribute to a series of molecular events leading to cellular senescence and a segmental progeroid syndrome.







Similar content being viewed by others
Data availability
Data that support the findings of this study are available from the corresponding author upon request.
References
Hisama FM, Oshima J, Martin GM. How research on human progeroid and antigeroid syndromes can contribute to the Longevity Dividend Initiative. Cold Spring Harb Perspect Med. 2016;6(4):a025882.
Gürsoy S, Hazan F, Öztürk T, Ateş H. Novel ocular and inner ear anomalies in a patient with Myhre syndrome. Mol Syndromol. 2020;10(6):339–43.
Oshima J, Sidorova JM, Monnat RJ Jr. Werner syndrome: clinical features, pathogenesis and potential therapeutic interventions. Ageing Res Rev. 2017;33:105–14.
Le Goff C, Mahaut C, Abhyankar A, Le Goff W, Serre V, Afenjar A, et al. Mutations at a single codon in Mad homology 2 domain of SMAD4 cause Myhre syndrome. Nat Genet. 2011;44(1):85–8.
Caputo V, Bocchinfuso G, Castori M, Traversa A, Pizzuti A, Stella L, et al. Novel SMAD4 mutation causing Myhre syndrome. Am J Med Genet A. 2014;164A(7):1835–40.
Burglen L, Heron D, Moerman A, Dieux-Coeslier A, Bourguignon JP, Bachy A, et al. Myhre syndrome: new reports, review, and differential diagnosis. J Med Genet. 2003;40(7):546–51.
Meerschaut I, Beyens A, Steyaert W, De Rycke R, Bonte K, De Backer T, et al. Myhre syndrome: a first familial recurrence and broadening of the phenotypic spectrum. Am J Med Genet A. 2019;179(12):2494–9.
Michot C, Le Goff C, Mahaut C, Afenjar A, Brooks AS, Campeau PM, et al. Myhre and LAPS syndromes: clinical and molecular review of 32 patients. Eur J Hum Genet. 2014;22(11):1272–7.
Miyazawa K, Miyazono K (2017) Regulation of TGF-beta family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol. 9(3)
Sugimoto M. A cascade leading to premature aging phenotypes including abnormal tumor profiles in Werner syndrome (review). Int J Mol Med. 2014;33(2):247–53.
Tang W, Robles AI, Beyer RP, Gray LT, Nguyen GH, Oshima J, et al. The Werner syndrome RECQ helicase targets G4 DNA in human cells to modulate transcription. Hum Mol Genet. 2016;25(10):2060–9.
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–68.
Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–46.
Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, Tchkonia T, Kirkland JL, Schwartz S. TNFalpha-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging (Albany NY). 2017;9(11):2411–35.
Lyu G, Guan Y, Zhang C, Zong L, Sun L, Huang X, et al. Addendum: TGF-beta signaling alters H4K20me3 status via miR-29 and contributes to cellular senescence and cardiac aging. Nat Commun. 2018;9(1):4134.
Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307(1):1–14.
Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19(8):1745–54.
Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700.
Morikawa M, Derynck R, Miyazono K (2016) TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 8(5)
Tominaga K, Suzuki HI (2019) TGF-beta Signaling in Cellular Senescence and Aging-Related Pathology. Int J Mol Sci. 20(20)
Hubackova S, Kucerova A, Michlits G, Kyjacova L, Reinis M, Korolov O, et al. IFNgamma induces oxidative stress, DNA damage and tumor cell senescence via TGFbeta/SMAD signaling-dependent induction of Nox4 and suppression of ANT2. Oncogene. 2016;35(10):1236–49.
Krieglstein K, Miyazono K, ten Dijke P, Unsicker K. TGF-beta in aging and disease. Cell Tissue Res. 2012;347(1):5–9.
Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347(1):155–75.
Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14(1):67–79.
Huang S, Lee L, Hanson NB, Lenaerts C, Hoehn H, Poot M, et al. The spectrum of WRN mutations in Werner syndrome patients. Hum Mutat. 2006;27(6):558–67.
Mori T, Yousefzadeh MJ, Faridounnia M, Chong JX, Hisama FM, Hudgins L, et al. ERCC4 variants identified in a cohort of patients with segmental progeroid syndromes. Hum Mutat. 2018;39(2):255–65.
Rubio MA, Kim SH, Campisi J. Reversible manipulation of telomerase expression and telomere length. Implications for the ionizing radiation response and replicative senescence of human cells. J Biol Chem. 2002;277(32):28609–17.
Saha B, Cypro A, Martin GM, Oshima J. Rapamycin decreases DNA damage accumulation and enhances cell growth of WRN-deficient human fibroblasts. Aging Cell. 2014;13(3):573–5.
Epstein CJ, Martin GM, Schultz AL, Motulsky AG. Werner’s syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore). 1966;45(3):177–221.
Oshima J, Campisi J, Tannock TC, Martin GM. Regulation of c-fos expression in senescing Werner syndrome fibroblasts differs from that observed in senescing fibroblasts from normal donors. J Cell Physiol. 1995;162(2):277–83.
Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell metabolism. 2013;17(5):644–56.
Yang X, Boehm JS, Salehi-Ashtiani K, Hao T, Shen Y, Lubonja R, et al. A public genome-scale lentiviral expression library of human ORFs. Nat Methods. 2011;8(8):659–61.
Saha B, Zitnik G, Johnson S, Nguyen Q, Risques RA, Martin GM, et al. DNA damage accumulation and TRF2 degradation in atypical Werner syndrome fibroblasts with LMNA mutations. Front Genet. 2013;4:129.
Fiorillo C, D'Apice MR, Trucco F, Murdocca M, Spitalieri P, Assereto S, et al. Characterization of MDPL Fibroblasts carrying the recurrent p.Ser605del mutation in POLD1 gene. DNA Cell Biol. 2018;37(12):1061–7.
Lessel D, Hisama FM, Szakszon K, Saha B, Sanjuanelo AB, Salbert BA, et al. POLD1 Germline mutations in patients initially diagnosed with Werner syndrome. Hum Mutat. 2015;36(11):1070–9.
Lessel D, Vaz B, Halder S, Lockhart PJ, Marinovic-Terzic I, Lopez-Mosqueda J, et al. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet. 2014;46(11):1239–44.
Sargolzaeiaval F, Zhang J, Schleit J, Lessel D, Kubisch C, Precioso DR, et al. CTC1 mutations in a Brazilian family with progeroid features and recurrent bone fractures. Mol Genet Genomic Med. 2018;6(6):1148–56.
Ioannidou A, Goulielmaki E, Garinis GA. DNA Damage: from chronic inflammation to age-related deterioration. Front Genet. 2016;7:187.
Martin GM, Sprague CA, Epstein CJ. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970;23(1):86–92.
Ghosh AK, Yuan W, Mori Y, Chen S, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J Biol Chem. 2001;276(14):11041–8.
Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397(6721):710–3.
Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–9.
von Zglinicki T, Saretzki G, Ladhoff J. d'Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126(1):111–7.
Wu L, Derynck R. Essential role of TGF-beta signaling in glucose-induced cell hypertrophy. Dev Cell. 2009;17(1):35–48.
Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3(1):37–48.
Magdalon J, Festuccia WT. Regulation of adiposity by mTORC1. Einstein (Sao Paulo). 2017;15(4):507–11.
Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet. 2004;363(9412):852–9.
Martin GM, Poot M, Haaf T. Lessons for aging from Werner syndrome epigenetics. Aging (Albany NY). 2020;12(3):2022–3.
Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389(6651):631–5.
Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–29.
Lin AE, Alali A, Starr LJ, Shah N, Beavis A, Pereira EM, et al. Gain-of-function pathogenic variants in SMAD4 are associated with neoplasia in Myhre syndrome. Am J Med Genet A. 2020;182(2):328–37.
Kim KS, Kang KW, Seu YB, Baek SH, Kim JR. Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech Ageing Dev. 2009;130(3):179–88.
Zhang Y, Alexander PB, Wang XF (2017) TGF-beta family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol. 9(4)
Eickelberg O, Pansky A, Koehler E, Bihl M, Tamm M, Hildebrand P, et al. Molecular mechanisms of TGF-(beta) antagonism by interferon (gamma) and cyclosporine A in lung fibroblasts. FASEB J. 2001;15(3):797–806.
Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172(3):1848–55.
Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, et al. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18(2):61–4.
Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol. 2000;149(3):667–82.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Acknowledgments
The authors are grateful to Ms. Lin Lee for administrative and laboratory assistance, Ms. Nino Giorgadze for isolation of primary human preadipocytes, and Dr. Tamar Pirtskhalava for insightful discussions.
Funding
This work was supported in part by JSPS KAKENHI 17H04037 (JO) and NIH grants R01CA210916 (GMM/JO), R37AG013925 (JLK/TT), and P01AG062413 (JLK/TT), and Robert and Arlene Kogod (JLK/TT), the Connor Group (JLK/TT), Robert J. and Theresa W. Ryan (JLK/TT), and the Noaber Foundation (JLK/TT).
Author information
Authors and Affiliations
Contributions
RKP designed and performed experiments, analyzed data, and wrote the manuscript. DH, JZ, and YY performed experiments and analyzed the data. GP and FH contributed clinical description of the patients and interpretation of clinical data. TM conducted exome analysis. TT and JLK contributed preadipocyte studies, supervised the project, and prepared and revised the manuscript. GMM and JO supervised the project and designed the study and prepared and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 580 kb)
About this article
Cite this article
Kandhaya-Pillai, R., Hou, D., Zhang, J. et al. SMAD4 mutations and cross-talk between TGF-β/IFNγ signaling accelerate rates of DNA damage and cellular senescence, resulting in a segmental progeroid syndrome—the Myhre syndrome. GeroScience 43, 1481–1496 (2021). https://doi.org/10.1007/s11357-020-00318-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00318-6