Abstract
Previous epidemiological studies have linked short-term exposure to particulate matter with outpatient visits for respiratory diseases. However, evidence on ultrafine particle (UFP) is still scarce in China. To investigate the association between short-term UFP exposure and outpatient visits for respiratory diseases as well as the corresponding lag patterns, information on outpatient visits for main respiratory diseases during January 1, 2017, to December 31, 2019 was collected from electronic medical records of two large tertiary hospitals in Shanghai, China. Generalized additive models employing a Quasi-Poisson distribution were employed to investigate the relationships between UFP and respiratory diseases. We computed the percentage change and its corresponding 95% confidence interval (CI) for outpatient visits related to respiratory diseases per interquartile range (IQR) increase in UFP concentrations. Based on a total of 1,034,394 hospital visits for respiratory diseases in Shanghai, China, we found that the strongest associations of total UFP with acute upper respiratory tract infection (AURTI), bronchitis, chronic obstructive pulmonary disease (COPD), and pneumonia occurred at lag 03, 03, 0, and 03 days, respectively. Each IQR increase in the total UFP concentrations was associated with increments of 9.02% (95% CI: 8.64–9.40%), 3.94% (95% CI: 2.84–5.06%), 4.10% (95% CI: 3.01–5.20%), and 10.15% (95% CI: 9.32–10.99%) for AURTI, bronchitis, COPD, and pneumonia, respectively. Almost linear concentration–response relationship curves without apparent thresholds were observed between total UFP and outpatient-department visits for four respiratory diseases. Stratified analyses illustrated significantly stronger associations of total UFP with AURTI, bronchitis, and pneumonia among female patients, while that with COPD was stronger among male patients. After adjustment of criteria air pollutants, these associations all remained robust. This time-series study indicates that short-term exposure to UFP was associated with increased risk of hospital visits for respiratory diseases, underscoring the importance of reducing ambient UFP concentrations for respiratory diseases control and prevention.
Similar content being viewed by others
Introduction
Mounting epidemiological studies have proposed the impacts of ambient particulate matter (PM) exposure on diverse health outcomes, including central nervous system diseases, cardiovascular diseases, and respiratory diseases (Liu et al. 2019; Shin et al. 2022; Yin et al. 2020). Previous toxicological researches further indicated that ultrafine particle (UFP), defined as particle with an aerodynamic diameter equal to or less than 0.1 μm, could deposit in the lower airways and alveoli, intrude into the circulatory system, and translocate to other organs more easily (Leikauf et al. 2020), thereby posing greater health risks compared to larger particles such as fine particulate matter with diameters less than 2.5 μm and 10 μm, known as PM2.5 and PM10, respectively (Hennig et al. 2018; Kim et al. 2015; Ohlwein et al. 2019). Furthermore, UFP might have a stronger ability to transport toxic components because they have a larger surface area relative to their diameter (Hu et al. 2020). Ambient UFP concentrations in China have been reported to surpass those observed in developed countries (Chen et al. 2019). Meanwhile, the WHO Air Quality Guidelines updated in 2021 suggested enhanced monitoring of UFP in the future (WHO 2021). The health impacts of UFP, however, have gained attention since recent years and currently represent an emerging area of research in environmental epidemiology (Hennig et al. 2018; Wright et al. 2021; Zhang et al. 2022).
Respiratory diseases are one of the leading causes of premature human mortality on a global scale (Allinson et al. 2023). While a few investigations have established associations between acute exposure to UFP and elevated morbidity and mortality related to diverse diseases (Chen et al. 2016; Hennig et al. 2018; Stafoggia et al. 2017), existing evidence concerning the respiratory impacts of UFP appears inconclusive. Several studies have found associations between UFP and various respiratory diseases, including chronic obstructive pulmonary disease (COPD), pneumonia, asthma, bronchitis, and upper respiratory tract infections (Lanzinger et al. 2016, Li et al. 2021, Weichenthal et al. 2017). However, another study found that ultrafine particle number concentration (PNC) did not affect respiratory health outcomes in humans (Clifford et al. 2018). The inconclusive findings might result from the different definitions of UFP (Li et al. 2021, Wright et al. 2021). To our knowledge, only a limited number of researches have explored the impact of short-term exposure to UFP on outpatient visits for multiple respiratory diseases, particularly in cities with relatively severe air pollution problems. Moreover, the independent relationships between UFP and respiratory diseases warrant further elucidation, given the potential confounding effects from other criteria air pollutants (Samoli et al. 2016).
Thus, we conducted a time-series study to assess the relationships between short-term UFP exposure and four main respiratory diseases (i.e., COPD; pneumonia; bronchitis; and acute upper respiratory tract infection, AURTI) in Shanghai, China. Furthermore, the independence of these associations was explored with adjustment for co-pollutants, and potential effect modifiers were assessed through stratified analyses.
Methods
Health data collection
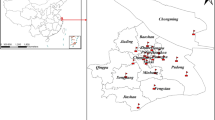
During the period from January 1, 2017, to December 31, 2019, we gathered comprehensive data on outpatients diagnosed with respiratory diseases from the electronic medical records of two prominent tertiary hospitals in Shanghai. To ensure standardization and uniformity in diagnosis coding, all recorded diagnoses were encoded according to the tenth version of the International Classification of Diseases (ICD-10). The essential patient data, including details like gender and age, onset time of initial symptoms, clinical diagnosis, results of clinical examinations, and treatment procedures, were all documented and inputted into the registry. All diagnoses were verified by specialists according to the Chinese Society of Respiratory Diseases criteria based on the symptoms and biochemical results. Outpatient visits for regular prescription and those who were transferred from other healthcare facilities were excluded to ensure a more focused and internally consistent study population. The study was approved by the Ethics Committee of the School of Public Health, Fudan University (IRB#2021–04-0889). Because the analysis was anonymous, informed permission was not required.
Environmental data
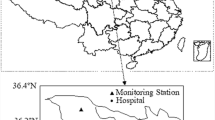
Daily UFP concentrations, measured in particles per cubic centimeter (particles/cm3), were collected from the Air Quality Monitoring Supersite of the Shanghai Environmental Monitoring Center (Fig. 1). A Scanning Mobility Particle Sizer manufactured by TSI, USA, was used to assess PNC within various size ranges (0.01–0.75 μm). To determine the concentration of total UFP (PNC0.01–0.10), the number concentrations of particles smaller than 0.10 μm in size were summed up. Additionally, particles ranging from 0.01 to 0.10 μm were further categorized into three smaller groups, specifically, 0.01 to 0.03 μm (PNC0.01–0.03), 0.03 to 0.05 μm (PNC0.03–0.05), and 0.05 to 0.10 μm (PNC0.05–0.10). The number concentrations of particles within the new range were obtained by summing up the readings for each of the initial finer size range. Daily concentrations of six criteria air pollutants including carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), PM2.5, and PM10 were extracted from the Shanghai Environmental Monitoring Center. All air quality monitoring stations in China were required to be located far from significant pollution sources, such as industrial facilities and traffic hubs. Therefore, pollution data collected in the present study could represent the background levels of the city. Furthermore, daily average meteorological factors (i.e., temperature and relative humidity) were gathered from the China Meteorological Data Service Center (http://data.cma.cn/en). Because there was only one available air quality monitoring supersite, all UFP concentrations in the present study were obtained from the same air quality monitoring supersite. Data on other criteria pollutants and meteorological factors were matched from the nearest monitoring station to the corresponding hospitals. Before conducting statistical analyses, extreme values were addressed by removing the maximum and minimum 0.1% of pollutant concentrations to mitigate the potential impact of outliers on the study results.
Statistical analyses
We adopted a time-series analytic approach to investigate the relationship between UFP exposure and respiratory diseases. To achieve this, we employed an overdispersed generalized additive model (GAM), a widely utilized method in previous researches to explore both nonlinear and lagged effects associated with short-term exposure to ambient air pollution. This approach is commonly utilized to investigate associations between air pollutant exposure and both mortality and morbidity (Coker et al. 2022; Peng et al. 2022; Ravindra et al. 2019; Zhang et al. 2021). We explored different lag structures, including current day (lag 0), the previous day (lag 1), 2 days before (lag 2), and 3 days before (lag 3). Additionally, we considered cumulated exposure over the concurrent day and the previous 1 (lag01), 2 (lag02), and 3 days (lag03), respectively. The daily average pollutant concentrations served as independent variables, while the number of daily visits for respiratory diseases was the dependent variable in the model. To account for potential confounding factors, several covariates were further included: (i) a natural spline function for time, using 7 degrees of freedom per year; (ii) a natural spline functions for present-day mean temperature and relative humidity, using 4 and 3 degrees of freedom, respectively; (iii) indicator variables for day of week (DOW) and public holidays (Jiang et al. 2023, Li et al. 2021, Peng et al. 2022).
The main model was:
where E(\({Y}_{i}\)) represents the estimated daily number of visits for respiratory diseases, \({Z}_{i}\) denotes the UFP concentration on day i. The regression coefficient for \({Z}_{i}\) is denoted by β, and α represents the intercept.
We plotted the concentration–response curves between UFP and respiratory diseases by introducing a natural cubic spline function with 5 degrees of freedom for UFP in the main model. To investigate potential effect modifiers, stratified analyses were conducted based on different variables. These variables included age groups categorized as 0–14, 15–44, 45–64, 65–74, and ≥ 75 years; sex (male and female); and seasons (warm: April − September and cold: October − March) (Li et al. 2023; Peng et al. 2022). To assess between-group differences, two-sample z-tests were employed using the following formula (Liu et al. 2021):
where β represents the estimates of different groups and its standard error SE.
To assess the robustness of the results, a series of sensitivity analyses were conducted. Firstly, we fitted multipollutant models by additionally controlling for the present-day (i.e., lag 0 day) and 3-day average (i.e., lag 03 day) concentrations of co-pollutants (PM2.5, PM10, NO2, SO2, CO, and O3) one by one and comparing the results from the co-pollutant model with those from the single pollutant model (Hu et al. 2020; Jiang et al. 2023). Secondly, we changed the number of degrees of freedom (from 5 to 9 per year) in the calendar time. Lastly, we adjusted for temperature and humidity averaged during the same lag period as UFP exposure in the model.
Data processing and statistical analyses were performed using R software (Version 4.2.3). All statistical tests were two-sided, with a significance level (α) set at 0.05. To assess the percent change in respiratory diseases visits associated with each interquartile range (IQR) increase in air pollution concentrations, odds ratios and their corresponding 95% confidence intervals (CI) were calculated using the following formulas:
Results
Descriptive results
From January 1, 2017, to December 31, 2019, a total of 1,034,394 hospital visits were recorded for respiratory diseases including 75,157 visits for COPD, 161,501 visits for pneumonia, 721,823 visits for AURTI, and 75,913 visits for bronchitis (Table 1). Of the patients, 50.85% were male and 43.89% aged under 15. Throughout the study period, the mean of various air pollutants was measured as follows: 3543 particles/cm3 for total UFP, 35.49 μg/m3 for PM2.5, 50.07 μg/m3 for PM10, 40.07 μg/m3 for NO2, 8.87 μg/m3 for SO2, 97.81 μg/m3 for O3, and 0.65 mg/m3 for CO (Table 2).
Table 3 and table S1 present correlations between meteorological and air pollution variables. Daily UFP concentrations displayed positive correlations with PM2.5 (Spearman r = 0.21, P < 0.001), PM10 (Spearman r = 0.35, P < 0.001), NO2 (Spearman r = 0.33, P < 0.001), SO2 (Spearman r = 0.36, P < 0.001), O3 (Spearman r = 0.18, P < 0.001), CO (Spearman r = 0.17, P < 0.001), and temperature (Spearman r = 0.06, P = 0.073), and were inversely related to relative humidity (Spearman r = -0.25, P < 0.001).
Regression results
Table 4 displays the percentage changes in daily outpatient visits for respiratory diseases linked to an IQR increase in total UFP concentrations across different lag periods. Generally, there were significant associations of total UFP with AURTI, bronchitis, COPD, and pneumonia. The lag patterns varied across different respiratory diseases. The strongest associations of total UFP with AURTI, bronchitis, COPD, and pneumonia occurred at lag 03, 03, 0, and 03 days, respectively. Each IQR increase in the total UFP concentrations was associated with increments of 9.02% (95% CI: 8.64–9.40%), 3.94% (95% CI: 2.84–5.06%), 4.10% (95% CI: 3.01–5.20%), and 10.15% (95% CI: 9.32–10.99%) in outpatient visits for AURTI, bronchitis, COPD, and pneumonia, respectively. We thus selected lag 03 days as the main lag for analyses of AURTI, bronchitis, and pneumonia, and lag 0 d for COPD.
Nearly linear concentration–response curves were observed between total UFP concentrations and outpatient-department visits for the four respiratory diseases, without apparent thresholds (Fig. 2). The concentration–response curve increased at lower concentrations and then became flat for AURTI. For bronchitis, COPD, and pneumonia, the concentration–response curves leveled off at lower concentrations and then slowly increased, with inflection points at approximately 4500, 3500, and 3000 particles/cm3, respectively.
Concentration–response curves for the predominant respiratory diseases associated with UFP concentrations. The lag periods in the analyses were lag 03 days for AURTI, bronchitis, and pneumonia, and lag 0 days for COPD. The solid lines represent mean estimates, and the shadow represents their 95% confidence intervals
Table 5 demonstrates the relationship between total UFP and daily outpatient visits for the overall respiratory diseases as well as four major subtypes stratified by age, sex, and season. We found that the associations of total UFP with AURTI, bronchitis, and pneumonia were the strongest in those aged 65–74 years, while that with COPD was the strongest in those aged 0–14. Notably, the differences were statistically significant across different age groups. The estimated effects of total UFP on AURTI were significantly stronger in female patients (9.57%, 95%CI: 9.04–10.11%) and in the cold seasons (15.10%, 95%CI: 14.50–15.69%) than in male patients (8.46%, 95%CI:7.93–9.00%) and in the warm seasons (1.95%, 95%CI: 1.45–2.45%), respectively. In contrast, for bronchitis, the risk was higher in females and in the warm season, but the differences were statistically significant only when comparing the groups stratified by sex. Besides, stronger associations between total UFP and COPD were observed in the cold season (4.83%, 95%CI: 3.32–6.46%) and in male patients (4.72%, 95% CI: 3.43–6.02%). For the association of total UFP with the visits of pneumonia, there was a significant effect modification by sex and season, with higher effect estimates observed in female and in the cold season.
Tables S2 and S3 show the relationship between outpatient visit for four respiratory diseases and total UFP exposure in co-pollutant models for the same exposure window (lag 03 days and lag 0 days, respectively). Results from the two-pollutant models remain robust and statistically significant, compared with those from the main model. For example, the estimated percent changes in the risk associated with UFP exposure at lag 03 days in the main model were 9.02, 3.94, 2.09, and 10.15 for AURTI, bronchitis, COPD, and pneumonia, respectively. In contrast, the effect estimates derived from two-pollutant models ranged from 7.51 to 9.11 for AURTI, 2.69 to 3.89 for bronchitis, 1.17 to 2.16 for COPD, and 9.56 to 10.61 for pneumonia, respectively. However, it is worth mentioning that when adjusting for PM2.5, PM10, SO2, and CO, the effects of total UFP on respiratory diseases showed a slight reduction. Despite the slight changes, all the associations generally remained significant. Results from other sensitivity analyses also remain stable (Table S4 and S5).
In Figure S1, the estimated percent change (%) and corresponding 95% CIs are depicted, illustrating the risk of respiratory diseases visits associated with each IQR increase in PNC. There was a significant association between daily respiratory disease visits and PNC of different particle sizes. Specifically, for AURTI, bronchitis, and pneumonia, larger particle sizes of ultrafine particulate matter produced the highest effect estimates on health. For COPD, on the other hand, there were comparable health effects of ultrafine particulate matter within different size ranges.
Discussion
The present study conducted a time-series analysis using daily UFP concentrations and outpatient visits for respiratory diseases in China. The risk of four main respiratory diseases consistently increases throughout the entire range of UFP concentrations. After adjusting for additional criterion pollutants, the results remained constant. Significantly higher effect estimates among women and during the cold season were observed. This study conducted a comprehensive and thorough assessment and provided compelling evidence on the respiratory impacts of short-term UFP exposure.
It has been demonstrated that ambient PM is an environmental risk factor for respiratory diseases. However, the evidence on the relationships between UFP and respiratory diseases is mixed. Numerous investigations have demonstrated that when UFP concentration increases, the risk of respiratory diseases also elevates noticeably (Belleudi et al. 2010, Lanzinger et al. 2016, Li et al. 2021, Mocelin et al. 2022, Weichenthal et al. 2017). For example, a time-series study conducted across 66 hospitals in Shanghai, China, demonstrated that the relative risk of outpatient visits for asthma, bronchitis, and upper respiratory tract infections increased with an IQR increase in UFP (1.21, 1.20, and 1.17, respectively) (Li et al. 2022). Another time-series study in China also showed that an IQR increase in UFP concentrations corresponded to elevated relative risks of emergency-department visits for various respiratory diseases, specifically, 1.35 for asthma, 1.20 for pneumonia, 1.17 for bronchitis, and 1.14 for upper respiratory tract infections (Li et al. 2021). However, these studies were limited to one or a few hospitals. By virtue of the time-series study design, the current analysis yields compelling evidence regarding significant relationship between short-term UFP exposure and the visits of respiratory diseases during various lag periods.
Several researches in the last few decades have also employed a time-series approach to investigate the relationship between PM and respiratory diseases (Coker et al. 2022; Kangas et al. 2023; Renzi et al. 2022). However, their sample sizes were notably smaller than ours, and the majority was carried out in developed nations characterized by lower ambient air pollution levels. Additionally, several earlier studies exclusively examined one specific form of respiratory disease (usually on COPD), raising questions of publication bias (Chen et al. 2021; Han et al. 2020; Wright et al. 2021). The current study offers robust and compelling evidence on the elevated incidence of all four respiratory diseases (i.e., AURTI, pneumonia, bronchitis, and COPD) associated with exposure to UFP. The evidence stems from a comprehensive investigation conducted in a sizable population residing in a major Chinese metropolis characterized by substantially higher levels of air pollution.
When evaluating the health impacts of UFP, the independence of the effects is also a key issue because earlier researches have not reached reliable results yet. Some researchers have found significant relationships between UFP and respiratory disease mortality or morbidity after adjusting for co-pollutants (Mocelin et al. 2022; Weichenthal et al. 2017), whereas other studies found a substantial attenuation in the relationships (Breitner et al. 2021; Lanzinger et al. 2016, Li et al. 2021). In multi-pollutant models, the present study found robust associations between the visits of respiratory diseases and UFP. The findings of our study offer initial evidence indicating the independent effects of UFP on respiratory health. Nevertheless, further studies are necessary to confirm these observations. The present results advocate for the recognition of UFP as a vital air pollutant deserving regular monitoring and emphasize the importance of implementing targeted UFP control strategies to mitigate the associated adverse respiratory effects.
We also investigated the exposure–response relationship and lagged associations between air pollution and visits for respiratory diseases. Notably, we observed slight variations in the shape of concentration–response curves and lag patterns across different respiratory diseases. The reasons for these variations remain uncertain and could potentially be attributed to distinct biological mechanisms. For example, the health impacts of UFP are closely linked to their ability to penetrate into circulatory system (da Costa et al. 2019). However, to comprehensively elucidate these biological mechanisms, future researches should encompass in vitro and in vivo toxicological studies as well as large-scale epidemiological investigations. These combined efforts will be instrumental in shedding light on the intricate pathways through which UFPs influence respiratory health.
The observed stronger associations of respiratory diseases visits among elderly patients and children can be attributed to their relatively weaker immune responses compared to other age groups (Belleudi et al. 2010; Lv et al. 2023; Peng et al. 2022). Furthermore, the higher incidence of pre-existing respiratory diseases in the elderly might have contributed to the stronger associations (Xu et al. 2016). Our findings were echoed by several previous studies that have indicated higher risk for respiratory diseases in women (Lv et al. 2023; Weichenthal et al. 2017). However, there are other studies showing higher vulnerability in males or observing no sex differences (Li et al. 2021, Mocelin et al. 2022). It should be noted that these results were based on limited sample sizes, and thus should be interpreted with caution.
Furthermore, we observed significantly higher effect estimates of UFP on respiratory diseases in the cold seasons compared to warm seasons. It is yet unclear how changes in season and temperature may affect the relationships between particulate matter and respiratory diseases. In the past, some researchers have found that hot weather increases the risk of respiratory diseases and death (Grigorieva and Lukyanets 2021, Lv et al. 2023), but other studies found that these relationships are higher in the winter or under cooler temperatures (Belleudi et al. 2010; Mocelin et al. 2022; Peng et al. 2022). Our research offers strong evidence for significantly higher effect estimates for UFP in cold seasons based on larger sample sizes. Several biological mechanisms may help to explain the seasonal differences. Low temperatures can pose direct adverse impacts on the pathogenesis of respiratory infections by influencing various inflammatory pathways and pathophysiological responses. These include alterations in the respiratory mucosa, such as vasoconstriction, as well as suppression of immune responses (Buckley and Richardson 2012, Gordon 2003; Graudenz et al. 2006). Thus, in order to reduce the prevalence of respiratory infections, it is essential to strengthen the monitoring and reporting of extreme temperatures and promote effective strategies to protect vulnerable populations when the air pollution levels are high.
Several hypothesized pathways might help to explain the acute relationship between UFP and respiratory disease visits (Leikauf et al. 2020). Due to its very small size, UFP could easily accumulate in the airways and alveoli. Previous evidence has shown that UFP is capable of transporting a wide range of hazardous materials, including transition metals, oxidizing gases, and organic compounds (Oberdorster 2001). All of these components have the potential to cause inflammation (Song et al. 2023; Wang et al. 2023, 2022a), which could induce and provoke respiratory diseases like COPD. Besides, other studies indicate that adverse respiratory effects associated with PM may also arise from the generation of reactive oxygen species and oxidative stress, which could case oxidant-mediated cellular damage to respiratory tract (Nel et al. 2006) and could further impact innate and adaptive immunity (Liu et al. 2023; Villegas et al. 2014; Wang et al. 2022b). However, to better elucidate this matter, future researches are still required.
The study had some limitations that need to be addressed. Firstly, the inclusion of data solely from Shanghai due to the unavailability of UFP concentration data from other cities might reduce the representativeness of the study population and potentially limit the generalizability of the findings to other locations. Secondly, it should be admitted that misclassification of exposure assessments was unavoidable because UFP data were obtained from a fixed-site air quality monitoring station, which was consistent with previous investigations (Hu et al. 2020, Li et al. 2021). However, such misclassification is considered as non-differential and could only lead to underestimated associations (Zeger et al. 2000).
Conclusions
In conclusion, this time-series analysis presents compelling evidence on the significant relationships between ambient UFP and increased risks of respiratory diseases. The associations were generally stronger in females and during cold seasons. These findings added to the limited knowledge on the detrimental health impacts of UFP and shed light on the temporal patterns of these effects. This study further emphasizes concerted efforts to address UFP pollution and implement targeted interventions for respiratory disease prevention and control.
Data availability
Access to the dataset could be available from the corresponding authors upon reasonable request.
References
Allinson JP, Chaturvedi N, Wong A, Shah I, Donaldson GC, Wedzicha JA, Hardy R (2023) Early childhood lower respiratory tract infection and premature adult death from respiratory disease in Great Britain: a national birth cohort study. Lancet 401:1183–1193
Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, Forastiere F (2010) Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 21:414–423
Breitner S, Su C, Franck U, Wiedensohler A, Cyrys J, Pan X, Wichmann HE, Schneider A, Peters A (2021) The Association Between Particulate Air Pollution and Respiratory Mortality in Beijing Before, During, and After the 2008 Olympic and Paralympic Games. Frontiers in Environmental Science 9:624180. https://doi.org/10.3389/fenvs.2021.624180
Buckley JP, Richardson DB (2012) Seasonal modification of the association between temperature and adult emergency department visits for asthma: a case-crossover study. Environ Health 11:55
Chen R, Hu B, Liu Y, Xu J, Yang G, Xu D, Chen C (2016) Beyond PM2.5: the role of ultrafine particles on adverse health effects of air pollution. Biochim Biophys Acta 1860:2844–2855
Chen Z, Fu Q, Mao G, Wu L, Xu P, Xu D, Wang Z, Pan X, Chen Y, Lou X, Mo Z, Wang X, Feng Y (2021) Increasing mortality caused by chronic obstructive pulmonary disease (COPD) in relation with exposure to ambient fine particulate matters: an analysis in Southeastern China. Environ Sci Pollut Res Int 28:53605–53613
Chen C, Ji W, Zhao B (2019) Size-dependent efficiencies of ultrafine particle removal of various filter media. Build Environ 160:106171. https://doi.org/10.1016/j.buildenv.2019.106171
Clifford S, Mazaheri M, Salimi F, Ezz WN, Yeganeh B, Low-Choy S, Walker K, Mengersen K, Marks GB, Morawska L (2018) Effects of exposure to ambient ultrafine particles on respiratory health and systemic inflammation in children. Environ Int 114:167–180
Coker ES, Buralli R, Manrique AF, Kanai CM, Amegah AK, Gouveia N (2022) Association between PM(2.5) and respiratory hospitalization in Rio Branco, Brazil: demonstrating the potential of low-cost air quality sensor for epidemiologic research. Environ Res 214:113738
da Costa EOJR, Base LH, de Abreu LC, Filho CF, Ferreira C, Morawska L (2019) Ultrafine particles and children’s health: literature review. Paediatr Respir Rev 32:73–81
Gordon CJ (2003) Role of environmental stress in the physiological response to chemical toxicants. Environ Res 92:1–7
Graudenz GS, Landgraf RG, Jancar S, Tribess A, Fonseca SG, Fae KC, Kalil J (2006) The role of allergic rhinitis in nasal responses to sudden temperature changes. J Allergy Clin Immunol 118:1126–1132
Grigorieva E, Lukyanets A (2021) Combined effect of hot weather and outdoor air pollution on respiratory health: literature review. Atmos 12:790. https://doi.org/10.3390/atmos12060790
Han C, Oh J, Lim YH, Kim S, Hong YC (2020) Long-term exposure to fine particulate matter and development of chronic obstructive pulmonary disease in the elderly. Environ Int 143:105895
Hennig F, Quass U, Hellack B, Kupper M, Kuhlbusch TAJ, Stafoggia M, Hoffmann B (2018) Ultrafine and fine particle number and surface area concentrations and daily cause-specific mortality in the Ruhr Area, Germany, 2009–2014. Environ Health Perspect 126:027008
Hu J, Tang M, Zhang X, Ma Y, Li Y, Chen R, Kan H, Cui Z, Ge J (2020) Size-fractionated particulate air pollution and myocardial infarction emergency hospitalization in Shanghai, China. Sci Total Environ 737:140100
Jiang Y, Chen R, Peng W, Luo Y, Chen X, Jiang Q, Han B, Su G, Duan Y, Huo J, Qu X, Fu Q, Kan H (2023) Hourly ultrafine particle exposure and acute myocardial infarction onset: an individual-level case-crossover study in Shanghai, China, 2015–2020. Environ Sci Technol 57:1701–1711
Kangas T, Gadeyne S, Lefebvre W, Vanpoucke C, Rodriguez-Loureiro L (2023) Are air quality perception and PM(2.5) exposure differently associated with cardiovascular and respiratory disease mortality in Brussels? Findings from a census-based study. Environ Res 219:115180
Kim KH, Kabir E, Kabir S (2015) A review on the human health impact of airborne particulate matter. Environ Int 74:136–143
Lanzinger S, Schneider A, Breitner S, Stafoggia M, Erzen I, Dostal M, Pastorkova A, Bastian S, Cyrys J, Zscheppang A, Kolodnitska T, Peters A, group Us (2016) Ultrafine and fine particles and hospital admissions in Central Europe. results from the UFIREG Study. Am J Respir Crit Care Med 194:1233–1241
Leikauf GD, Kim SH, Jang AS (2020) Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med 52:329–337
Li H, Liu L, Chen R, Feng R, Zhou Y, Hong J, Cao L, Lu Y, Dong X, Xia M, Ding B, Weng Y, Qian L, Wang L, Zhou W, Gui Y, Han X, Zhang X (2022) Size-segregated particle number concentrations and outpatient-department visits for pediatric respiratory diseases in Shanghai, China. Ecotoxicol Environ Saf 243:113998
Li H, Li X, Zheng H, Liu L, Wu Y, Zhou Y, Meng X, Hong J, Cao L, Lu Y, Dong X, Xia M, Ding B, Qian L, Wang L, Zhou W, Gui Y, Kan H, Chen R, Zhang X (2021) Ultrafine particulate air pollution and pediatric emergency-department visits for main respiratory diseases in Shanghai, China. Sci Total Environ 775:145777. https://doi.org/10.1016/j.scitotenv.2021.145777
Li H, Liang L, Zhang S, Qian Z, Cai M, Wang X, McMillin SE, Keith AE, Wei J, Geng Y, Lin H (2023) Short-term ambient particulate matter pollution of different sizes and respiratory hospital admission in the Beibu Gulf area of Southern China. Atmos Environ 294:119524. https://doi.org/10.1016/j.atmosenv.2022.119524
Liu C et al (2019) Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381:705–715
Liu Y, Pan J, Fan C, Xu R, Wang Y, Xu C, Xie S, Zhang H, Cui X, Peng Z, Shi C, Zhang Y, Sun H, Zhou Y, Zhang L (2021) Short-term exposure to ambient air pollution and mortality from myocardial infarction. J Am Coll Cardiol 77:271–281
Liu W, Yu L, Zhou M, Ye Z, Liang R, Tan Q, Song J, Ma J, Wang D, Wang B, Chen W (2023) Cross-sectional and longitudinal associations between propylene oxide exposure and lung function among Chinese community residents: roles of oxidative DNA damage, lipid peroxidation, and protein carbonylation. Chest 163:1395–1409
Lv S, Liu X, Li Z, Lu F, Guo M, Liu M, Wei J, Wu Z, Yu S, Li S, Li X, Gao W, Tao L, Wang W, Xin J, Guo X (2023) Causal effect of PM(1) on morbidity of cause-specific respiratory diseases based on a negative control exposure. Environ Res 216:114746
Mocelin HT, Fischer GB, Bush A (2022) Adverse early-life environmental exposures and their repercussions on adult respiratory health. J Pediatr (rio j) 98(Suppl 1):S86–S95
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Oberdorster G (2001) Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health 74:1–8
Ohlwein S, Kappeler R, Kutlar Joss M, Kunzli N, Hoffmann B (2019) Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int J Public Health 64:547–559
Peng W, Li H, Peng L, Wang Y, Wang W (2022) Effects of particulate matter on hospital admissions for respiratory diseases: an ecological study based on 12.5 years of time series data in Shanghai. Environ Health 21:12. https://doi.org/10.1186/s12940-021-00828-6
Ravindra K, Rattan P, Mor S, Aggarwal AN (2019) Generalized additive models: building evidence of air pollution, climate change and human health. Environ Int 132:104987
Renzi M et al (2022) A nationwide study of air pollution from particulate matter and daily hospitalizations for respiratory diseases in Italy. Sci Total Environ 807:151034
Samoli E, Andersen ZJ, Katsouyanni K, Hennig F, Kuhlbusch TA, Bellander T, Cattani G, Cyrys J, Forastiere F, Jacquemin B, Kulmala M, Lanki T, Loft S, Massling A, Tobias A, Stafoggia M, Uf, group HS (2016) Exposure to ultrafine particles and respiratory hospitalisations in five European cities. Eur Respir J 48:674–82
Shin HH, Maquiling A, Thomson EM, Park IW, Stieb DM, Dehghani P (2022) Sex-difference in air pollution-related acute circulatory and respiratory mortality and hospitalization. Sci Total Environ 806:150515
Song J, Wang D, Zhou M, You X, Tan Q, Liu W, Yu L, Wang B, Chen W, Zhang X (2023) Carbon disulfide exposure induced lung function reduction partly through oxidative protein damage: a cross-sectional and longitudinal analysis. J Hazard Mater 454:131464
Stafoggia M et al (2017) Association between short-term exposure to ultrafine particles and mortality in eight European urban areas. Epidemiology 28:172–180
Villegas L, Stidham T, Nozik-Grayck E (2014) Oxidative Stress and therapeutic development in lung diseases. J Pulm Respir Med 4:194. https://doi.org/10.4172/2161-105X.1000194
Wang B, Wang X, Yu L, Liu W, Song J, Fan L, Zhou M, Yang M, Ma J, Cheng M, Qiu W, Liang R, Wang D, Guo Y, Chen W (2022a) Acrylamide exposure increases cardiovascular risk of general adult population probably by inducing oxidative stress, inflammation, and TGF-beta1: a prospective cohort study. Environ Int 164:107261
Wang B, Yu L, Liu W, Yang M, Fan L, Zhou M, Ma J, Wang X, Nie X, Cheng M, Qiu W, Ye Z, Song J, Chen W (2022b) Cross-sectional and longitudinal associations of acrolein exposure with pulmonary function alteration: assessing the potential roles of oxidative DNA damage, inflammation, and pulmonary epithelium injury in a general adult population. Environ Int 167:107401
Wang B, Liu W, Yu L, Ye Z, Cheng M, Qiu W, Zhou M, Ma J, Wang X, Yang M, Song J, Chen W (2023) Acrolein exposure impaired glucose homeostasis and increased risk of type 2 diabetes: an urban adult population-based cohort study with repeated measures. Environ Sci Technol 57:7162–7173
Weichenthal S, Bai L, Hatzopoulou M, Van Ryswyk K, Kwong JC, Jerrett M, van Donkelaar A, Martin RV, Burnett RT, Lu H, Chen H (2017) Long-term exposure to ambient ultrafine particles and respiratory disease incidence in in Toronto, Canada: a cohort study. Environ Health 16:64
World Health Organisation (2021) WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Retrieved from https://iris.who.int/bitstream/handle/10665/345329/9789240034228-eng.pdf?sequence=1
Wright RJ, Hsu HL, Chiu YM, Coull BA, Simon MC, Hudda N, Schwartz J, Kloog I, Durant JL (2021) Prenatal ambient ultrafine particle exposure and childhood asthma in the Northeastern United States. Am J Respir Crit Care Med 204:788–796
Xu Q, Li X, Wang S, Wang C, Huang F, Gao Q, Wu L, Tao L, Guo J, Wang W, Guo X (2016) Fine particulate air pollution and hospital emergency room visits for respiratory disease in urban areas in Beijing, China, in 2013. PLoS One 11:e0153099
Yin P, Brauer M, Cohen AJ, Wang H, Li J, Burnett RT, Stanaway JD, Causey K, Larson S, Godwin W, Frostad J, Marks A, Wang L, Zhou M, Murray CJL (2020) The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990–2017: an analysis for the Global Burden of Disease Study 2017. Lancet Planet Health 4:e386–e398
Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A (2000) Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect 108:419–426
Zhang F, Zhang H, Wu C, Zhang M, Feng H, Li D, Zhu W (2021) Acute effects of ambient air pollution on clinic visits of college students for upper respiratory tract infection in Wuhan, China. Environ Sci Pollut Res Int 28:29820–29830
Zhang Y, He Q, Zhang Y, Xue X, Kan H, Wang X (2022) Differential associations of particle size ranges and constituents with stroke emergency-room visits in Shanghai, China. Ecotoxicol Environ Saf 232:113237
Acknowledgements
The authors would like to thank all the subjects who participated in this study.
Funding
This study was supported by the Minhang Natural Science Foundation of Shanghai, China (2021MHZ001).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yiqin Gu and Qingyan Fu are joint corresponding authors and contributed equally to this work. Material preparation, data collection, and analysis were performed by Ran Yan, Shengjie Ying, and Yixuan Jiang. The first draft of the manuscript was written by Ran Yan, Shengjie Ying, and Yixuan Jiang. Yusen Duan, Renjie Chen, and Haidong Kan provided data and contributed to the interpretation of the results and the submitted version of the manuscript. Yiqin Gu and Qingyan Fu conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the School of Public Health, Fudan University (IRB#2021–04-0889). Because the analysis was anonymous, informed permission was not required.
Consent for publication
The authors confirm:
• That the work described has not been published before
• That it is not under consideration for publication elsewhere
• That its publication has been approved by all co-authors, if any
• That its publication has been approved by the responsible authorities at the institution where the work is carried out.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ran Yan, Shengjie Ying, and Yixuan Jiang contributed equally to this work and should be considered as co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, R., Ying, S., Jiang, Y. et al. Associations between ultrafine particle pollution and daily outpatient visits for respiratory diseases in Shanghai, China: a time-series analysis. Environ Sci Pollut Res 31, 3004–3013 (2024). https://doi.org/10.1007/s11356-023-31248-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31248-3