Abstract
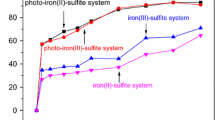
Fenton-like oxidation processes are widely used to degrade recalcitrant organic pollutants, but are limited by narrow application pH and low reaction efficiency. This study investigated the synchronous activation of H2O2 and persulfate (PDS) by sulfidated zero valent iron (S-nZVI) in ambient conditions for Fenton-like oxidation of bisphenol S (BPS), an estrogenic endocrine-disrupting chemical. The activation of S-nZVI induced H2O2 or PDS could be greatly enhanced with the assistance of PDS and H2O2, respectively, even across a wide range of pH value (3–11). The first-order rate constant of S-nZVI/H2O2/PDS, S-nZVI/PDS and S-nZVI/H2O2 systems was found to be 0.2766 min−1, 0.0436 min−1, and 0.0113 min−1, respectively. A significant synergy between H2O2 and PDS was achieved when the PDS-H2O2 molar ratio was above 1:1, and where sulfidation promoted iron corrosion and decreased solution pH were observed in the S-nZVI/H2O2/PDS system. Radical scavenging experiments and electron paramagnetic resonance (EPR) investigations suggest that both SO4•− and •OH were generated and that •OH played a crucial role in BPS removal. Furthermore, four BPS degradation intermediates were detected and three degradation pathways were proposed in line with the HPLC-Q-TOF–MS analysis. This study demonstrated that compared to the traditional Fenton-like system, the S-nZVI/H2O2/PDS system could be a more efficient, advanced oxidation technology capable of being used across a broad pH range for emerging pollutants’ degradation.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Alsbaiee A, Smith BJ, Xiao LL, Ling YH, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 529:190-U146. https://doi.org/10.1038/nature16185
Baravalle R, Ciaramella A, Baj F, Di Nardo G, Gilardi G (2018) Identification of endocrine disrupting chemicals acting on human aromatase. Bba-Proteins Proteom 1866:88–96. https://doi.org/10.1016/j.bbapap.2017.05.013
Brumovsky M, Oborna J, Lacina P, Hegedus M, Sracek O, Kolarik J, Petr M, Kaslik J, Hofmann T, Filip J (2021) Sulfidated nano-scale zerovalent iron is able to effectively reduce in situ hexavalent chromium in a contaminated aquifer. J Hazard Mater 405. https://doi.org/10.1016/j.jhazmat.2020.124665
Cao Z, Liu X, Xu J, Zhang J, Yang Y, Zhou JL, Xu XH, Lowry GV (2017) Removal of Antibiotic Florfenicol by Sulfide-Modified Nanoscale Zero-Valent Iron. Environ Sci Technol 51:11269–11277. https://doi.org/10.1021/acs.est.7b02480
Dong HR, Zhang C, Deng JM, Jiang Z, Zhang LH, Cheng YJ, Hou KJ, Tang L, Zeng GM (2018) Factors influencing degradation of trichloroethylene by sulfide-modified nanoscale zero-valent iron in aqueous solution. Water Res 135:1–10. https://doi.org/10.1016/j.watres.2018.02.017
Du JK, Wang Y, Faheem XuTT, Zheng H, Bao JG (2019) Synergistic degradation of PNP via coupling H2O2 with persulfate catalyzed by nano zero valent iron. Rsc Adv 9:20323–20331. https://doi.org/10.1039/c9ra02901j
Fan DM, Lan Y, Tratnyek PG, Johnson RL, Filip J, O’Carroll DM, Garcia AN, Agrawal A (2017) Sulfidation of Iron-Based Materials: A Review of Processes and Implications for Water Treatment and Remediation. Environ Sci Technol 51:13070–13085. https://doi.org/10.1021/acs.est.7b04177
Fang Z, Gao YT, Wu XL, Xu XY, Sarmah AK, Bolan N, Gao B, Shaheen SM, Rinklebe J, Ok YS, Xu S, Wang HL (2020) A critical review on remediation of bisphenol S (BPS) contaminated water: Efficacy and mechanisms. Crit Rev Env Sci Tec 50:476–522. https://doi.org/10.1080/10643389.2019.1629802
Gao QW, Ding J, Zhao GS, Zhao QL, Li LL, Zhao XS, Bu LJ, Zhou SQ, Qiu S (2022) Exploring the synergism of sunlight and electrooxidation on persulfate activation for efficient degradation of bisphenol S: Performance, Pathway, and mechanism. Chem Eng J 437. https://doi.org/10.1016/j.cej.2022.135318
Gong YY, Gai LS, Tang JC, Fu J, Wang QL, Zeng EY (2017) Reduction of Cr(VI) in simulated groundwater by FeS-coated iron magnetic nanoparticles. Sci Total Environ 595:743–751. https://doi.org/10.1016/j.scitotenv.2017.03.282
Grcic I, Vujevic D, Koprivanac N (2010) Modeling the mineralization and discoloration in colored systems by (US)Fe2+/H2O2/S2O82-processes: A proposed degradation pathway. Chem Eng J 157:35–44. https://doi.org/10.1016/j.cej.2009.10.042
Greenlee LF, Torrey JD, Amaro RL, Shaw JM (2012) Kinetics of Zero Valent Iron Nanoparticle Oxidation in Oxygenated Water. Environ Sci Technol 46:12913–12920. https://doi.org/10.1021/es303037k
Han YL, Yan WL (2016) Reductive Dechlorination of Trichloroethene by Zero-valent Iron Nanoparticles: Reactivity Enhancement through Sulfidation Treatment. Environ Sci Technol 50:12992–13001. https://doi.org/10.1021/acs.est.6b03997
He D, Ma JX, Collins RN, Waite TD (2016) Effect of Structural Transformation of Nanoparticulate Zero-Valent Iron on Generation of Reactive Oxygen Species. Environ Sci Technol 50:3820–3828. https://doi.org/10.1021/acs.est.5b04988
Ji L, Wang XD, Yang XS, Liu SS, Wang LS (2008) Back-propagation network improved by conjugate gradient based on genetic algorithm in QSAR study on endocrine disrupting chemicals. Chinese Sci Bull 53:33–39. https://doi.org/10.1007/s11434-007-0484-6
Kim EJ, Kim JH, Azad AM, Chang YS (2011) Facile Synthesis and Characterization of Fe/FeS Nanoparticles for Environmental Applications. Acs Appl Mater Inter 3:1457–1462. https://doi.org/10.1021/am200016v
Kim EJ, Kim JH, Chang YS, Turcio-Ortega D, Tratnyek PG (2014) Effects of Metal Ions on the Reactivity and Corrosion Electrochemistry of Fe/FeS Nanoparticles. Environ Sci Technol 48:4002–4011. https://doi.org/10.1021/es405622d
Knez J (2013) Endocrine-disrupting chemicals and male reproductive health. Reprod Biomed Online 26:440–448. https://doi.org/10.1016/j.rbmo.2013.02.005
Kuruto-Niwa R, Nozawa R, Miyakoshi T, Shiozawa T, Terao Y (2005) Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ Toxicol Phar 19:121–130. https://doi.org/10.1016/j.etap.2004.05.009
Li J, Xu MJ, Yao G, Lai B (2018) Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem Eng J 348:1012–1024. https://doi.org/10.1016/j.cej.2018.05.032
Liao CY, Liu F, Kannan K (2012) Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol A Residues. Environ Sci Technol 46:6515–6522. https://doi.org/10.1021/es300876n
Lu XH, Zeng YX, Yu MH, Zhai T, Liang CL, Xie SL, Balogun MS, Tong YX (2014) Oxygen-Deficient Hematite Nanorods as High-Performance and Novel Negative Electrodes for Flexible Asymmetric Supercapacitors. Adv Mater 26:3148–3155. https://doi.org/10.1002/adma.201305851
Mehrabani-Zeinabad M, Achari G, Langford CH (2016) Degradation of Bisphenol S Using O3 and/or H2O2 with UV in a Flow-Through Reactor. J Environ Eng 142. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001117
Mei Q, Sun JF, Han DN, Wei B, An ZX, Wang XY, Xie J, Zhan JH, He MX (2019) Sulfate and hydroxyl radicals-initiated degradation reaction on phenolic contaminants in the aqueous phase: Mechanisms, kinetics and toxicity assessment. Chem Eng J 373:668–676. https://doi.org/10.1016/j.cej.2019.05.095
Nakada N, Shinohara H, Murata A, Kiri K, Managaki S, Sato N, Takada H (2007) Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res 41:4373–4382. https://doi.org/10.1016/j.watres.2007.06.038
Ogata Y, Goda S, Toyama T, Sei K, Ike M (2013) The 4-tert-Butylphenol-Utilizing Bacterium Sphingobium fuliginis OMI Can Degrade Bisphenols via Phenolic Ring Hydroxylation and Meta-Cleavage Pathway. Environ Sci Technol 47:1017–1023. https://doi.org/10.1021/es303726h
Song SK, Su MM, Adeleye AS, Zhang YL, Zhou XF (2017) Optimal design and characterization of sulfide-modified nanoscale zerovalent iron for diclofenac removal. Appl Catal B-Environ 201:211–220. https://doi.org/10.1016/j.apcatb.2016.07.055
Sun YK, Li JX, Huang TL, Guan XH (2016) The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res 100:277–295. https://doi.org/10.1016/j.watres.2016.05.031
Tratnyek PG, Salter-Blanc AJ, Nurmi JT, Amonette JE, Liu J, Wang CM, Dohnalkova A, Baer DR (2011) Reactivity of Zerovalent Metals in Aquatic Media: Effects of Organic Surface Coatings. Acs Sym Ser 1071:381-+
Wang JJ, Liu BJ, Liu HF, Hu X, Zhou SQ (2022) Insight into the mechanisms of BPS degradation by electro-Fenton method modified by co-based nanoparticles on the oxidized carbon cathode. Chem Eng J 446. https://doi.org/10.1016/j.cej.2022.137376
Wang LL, Zhang ZZ, Zhang J, Zhang L (2016) Magnetic solid-phase extraction using nanoporous three dimensional graphene hybrid materials for high-capacity enrichment and simultaneous detection of nine bisphenol analogs from water sample. J Chromatogr A 1463:1–10. https://doi.org/10.1016/j.chroma.2016.08.003
Wei XP, Guo ZY, Yin H, Yuan YB, Chen RX, Lu GN, Dang Z (2021) Removal of heavy metal ions and polybrominated biphenyl ethers by sulfurized nanoscale zerovalent iron: Compound effects and removal mechanism. J Hazard Mater 414. https://doi.org/10.1016/j.jhazmat.2021.125555
Wu JX, Wang B, Cagnetta G, Huang J, Wang YJ, Deng SB, Yu G (2020) Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep Purif Technol 239. https://doi.org/10.1016/j.seppur.2020.116534
Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PKS, Moon HB, Jeong Y, Kannan P, Achyuthan H, Munuswamy N, Kannan K (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotox Environ Safe 122:565–572. https://doi.org/10.1016/j.ecoenv.2015.09.029
Funding
This work was supported by the National Key R&D Program of China (2021YFE0106600) and National Natural Science Foundations of China (41907153).
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation and supervision: Jiangkun Du, Liting Luo; investigation, data curation, writing – original draft and methodology: Yehan Xiong; validation: Ting Zhou; writing – review and editing: Yehan Xiong, Ting Zhou, Jianguo Bao, Muhammad Faheem.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, Y., Zhou, T., Bao, J. et al. Degradation mechanism of Bisphenol S via hydrogen peroxide/persulfate activated by sulfidated nanoscale zero valent iron. Environ Sci Pollut Res 30, 83545–83557 (2023). https://doi.org/10.1007/s11356-023-28189-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28189-2