Abstract
The COVID-19 pandemic resulted in the collapse of healthcare systems and led to the development and application of several approaches of wastewater-based epidemiology to monitor infected populations. The main objective of this study was to carry out a SARS-CoV-2 wastewater based surveillance in Curitiba, Southern Brazil Sewage samples were collected weekly for 20 months at the entrance of five treatment plants representing the entire city and quantified by qPCR using the N1 marker. The viral loads were correlated with epidemiological data. The correlation by sampling points showed that the relationship between the viral loads and the number of reported cases was best described by a cross-correlation function, indicating a lag between 7 and 14 days amidst the variables, whereas the data for the entire city presented a higher correlation (0.84) with the number of positive tests at lag 0 (sampling day). The results also suggest that the Omicron VOC resulted in higher titers than the Delta VOC. Overall, our results showed that the approach used was robust as an early warning system, even with the use of different epidemiological indicators or changes in the virus variants in circulation. Therefore, it can contribute to public decision-makers and health interventions, especially in vulnerable and low-income regions with limited clinical testing capacity. Looking toward the future, this approach will contribute to a new look at environmental sanitation and should even induce an increase in sewage coverage rates in emerging countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The fast dissemination of severe acute respiratory syndrome 2 (SARS-CoV-2), which causes a severe acute respiratory syndrome known as coronavirus disease (COVID-19), resulted in many countries' collapse of the healthcare system. The diffusion of COVID-19 within a community is influenced by various factors such as population density, the presence of new viral mutations, vaccination rates, and a range of geographic and environmental determinants. These determinants include aspects like air quality, UV radiation levels, and meteorological variables (Coccia 2020, 2022a; Barcellos et al. 2021; Magazzino et al. 2022; Mathys et al. 2023). Although risk factors and levels of preparedness to deal with COVID-19 cases vary among countries (Coccia 2022a), clinical testing and early diagnosis of COVID-19 cases are part of a strategy to combat the pandemic (Salath et al. 2020). However, clinical testing is expensive, time-consuming, and may be limited to people with symptoms (Pilevar et al. 2021). In this context, it has been insufficient mainly in underdeveloped and developing countries, which led to the investigation of several alternative tools to provide earlier information on COVID-19 cases to alert the population and support decision-makers. Although the most common clinical manifestations associated with COVID-19 include fever, cough, and dyspnea (Baj et al. 2020; da Rosa Mesquita et al. 2021), it has been shown that infected patients can present gastroenteric symptoms, with the virus genetic material detected in feces and urine (Xiao et al. 2020; Jones et al. 2020; Fumian et al. 2021). As the virus can be found in human excretions, it has been demonstrated that it is possible to detect the viral RNA in wastewater, and the quantification of this genetic material can act as an early warning system regarding the number of infected people with COVID-19 (Ahmed et al. 2020; Medema et al. 2020).
From the beginning of the COVID-19 pandemic, wastewater-based epidemiology (WBE) has been reported as a powerful complementary tool for examining an entire population or community and determining the effectiveness and direction of government actions on health (Rice et al. 2020). Wastewater sampling enables the collection of composite samples from entire populations, comprising a mixture of excretions from individuals in the community (Kumblathan et al. 2023), so this approach is not subjected to bias found in traditional epidemiological monitoring, which depends on the degree of access to health services and testing, and where all potential patients and asymptomatic cases cannot be accounted for (Mao et al. 2020). It has been demonstrated that SARS-CoV-2 might be transmitted to other people before the first symptoms appear (Rothan and Byrareddy 2020; Singhal 2020), with estimates that presymptomatic and asymptomatic cases can account for at least 50% of all transmission events (Johansson et al. 2021). Accordingly, as WBE does not depend on individual testing, this approach is highly suited as an early warning system for the fluctuations in circulating viral loads, with several studies showing that the increase or decrease in the viral load in wastewaters can be correlated to increases or decreases in the number of infected in subsequent days (Ahmed et al. 2020; Gerrity et al. 2021; Krivoňáková et al. 2021; Karthikeyan et al. 2021).
The methods for SARS-CoV-2 WBE vary in efficiency and sensitivity due to local settings and to different combinations of wastewater sampling strategies and RNA extraction and preservation techniques (Le 2023; Li et al. 2023a; Feng et al. 2023; Torabi et al. 2023). Nonetheless, they have been adopted successfully in several countries on all continents with human settlements (Street et al. 2020; Ahmed et al. 2020; Gonzalez et al. 2020; Arora et al. 2020; Agrawal et al. 2021b, a; Weidhaas et al. 2021; Bar-Or et al. 2021; Hasan et al. 2021; Saththasivam et al. 2021; Carrillo-Reyes et al. 2021; Wu et al. 2021; Yaniv et al. 2021; Giraud-Billoud et al. 2021). In Brazil, the monitoring and quantification of viral ribonucleic acid (RNA) as a tool for epidemiological monitoring was readily implemented in some cities, with published reports from Niteroi (Rio de Janeiro State) (Prado et al. 2020, 2021), São Paulo and adjacent cities (São Paulo State) (Claro et al. 2021; Razzolini et al. 2021; Barbosa et al. 2022; Bueno et al. 2022; Martins et al. 2022), Belo Horizonte (Minas Gerais State) (Chernicharo et al. 2020; Mota et al. 2021) and rural settings in southeastern Minas Gerais (Fongaro et al. 2021), Goiânia (Goiás State), the Federal District, and Foz do Iguaçu (Paraná State) (Bueno et al. 2022).
In Brazil, a team of scientists, professionals, students, and companies is working on the COVID Sewage Monitoring Network (https://etes-sustentaveis.org/rede-monitoramento-covid-esgotos/), which was created in March 2021, to provide additional information’s at different local levels about the COVID-19 pandemic. The project has been expanded to include the monitoring of sewage in six major Brazilian capitals, based on a methodology established in previous studies performed in Belo Horizonte in five state capitals and the federal capital of Brazil (Mota et al. 2021). The present study utilized data from a 20-month weekly WBE COVID-19 monitoring conducted at five distinct wastewater treatment plants (WTPs) in one of these capitals, Curitiba, the largest city in southern Brazil. The three main objectives of the present were to analyze if the number of daily reported cases by health regionals in different sectors of the city correlates with the viral loads found in the wastewater treatment plants (WWTPs) that serve each region; to access the trends of three different epidemiological indicators (daily number of positive tests, reported cases, and active cases) in relation to the weekly measured wastewater SARS-CoV-2 titers (combined data); and to examine if the waves caused by different SARS-CoV-2 variants of concern (VOC) lead to any changes in the wastewater viral titers measured during the monitoring.
Material and methods
Samples and data
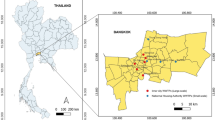
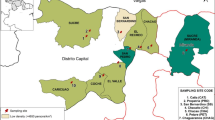
The city of Curitiba, the capital of the Paraná State, is located in southern Brazil, has a population of 1,948,626 inhabitants and is one of the Brazilian cities with the best sanitation system coverage, with approximately 95.1% of the population served by the sewage collection (SNIS 2020). The sewer network divides the city into five macro-regions, and all collected sewage is transported to five WWTPs, which treat 100% of the collected sewage, as presented in Fig. 1. In addition, these WWTPs also receive sewage from part of the metropolitan region of Curitiba, including cities with more than 100 thousand inhabitants, as São José dos Pinhais (WWTP Belém), Colombo, Pinhais, Piraquara (WWTP Atuba Sul), and Araucária (WWTP CIC-Xisto). SARS-CoV-2 RNA in the inlet of these WWTPs was monitored weekly from March 2021 to November 2022. The coverage area, flow rates, population served, covered cities, and the number of epidemiological weeks monitored of sampling points are shown in Table 1.
At each sampling point composite samples were obtained in the WWTPs a period of 4 h during the morning (from 8:00am to 12:00 pm) using automatic sampling devices (HACH AS950 and SD900), which were set to collect 400 mL of sewage every 10 min, resulting in approximately 9.60 L total volume. After sampling, the sample containers were vigorously shaken, and composite samples were transferred to two bottles of 1 L, which were cooled and immediately transported to the laboratory for SARS-CoV-2 RNA extraction and preservation. Upon arrival of the samples in the laboratory they were immediately processed and extracted at NB2 + cabinets. The concentration of the wastewater material was carried out using a methodology adapted from Symonds et al. (2014) and Ahmed et al. (2015), as described by Mota et al. (2021). The method is based on filtration of the sample through an electronegative membrane (HAWP04700, Merck-Millipore, cellulose ester membrane with pore diameter of 0.45 µm) using a vacuum pump. Before filtration, sample volumes of 100 mL were prepared by adding 1 mL of MgCl2 (2.5 M). After homogenization, they were acidified with acetic acid (2 M instead of 1 M as described by Mota et al. 2021) until they reached a pH between 3 and 3.5. Final acid volume added ranged from 0.002–0.033 L (mean = 0.005 ± 0.004 L, n = 458). Samples were filtered until membrane clogged, so the volume filtrated was variable, ranging from 0.028–0.1 L (mean = 0.063 ± 0.017 L, n = 458). After filtration, the membrane was transferred to a sterilized microtube and stored at -80 C° until extraction (typically between 24–48 h after filtering). The genetic material withheld on the membrane was extracted using the AllPrep PowerViral DNA/RNA extraction kit (Qiagen Inc., Düsseldorf, Germany) following the manufacturer's instructions, except that after the addition of the PM1 lysis buffer to the beads tube with the membrane, the membranes were manually fragmented using a pipet tip before being placed on the vortex adaptor. The final volume used for DNA/RNA elution was 100 µL.
COVID-19 clinical data for Curitiba City, including the number of positive tests per day, reported cases, active cases, and the social restrictions/isolation, were obtained from March 2021 to December 2022 through the COVID-19 Curitiba Panel (https://coronavirus.curitiba.pr.gov.br/painelcovid). Data for the number of reported cases in the different districts of Curitiba were obtained from the open database of COVID-19 cases (https://www.curitiba.pr.gov.br/dadosabertos/). Information on the SARS-CoV-2 variants of concern (VOC) in circulation were obtained from Adamoski et al. (2022, 2021) and from Paraná State Government press releases, with the first register of Omicron lineage BA.2 in the metropolitan region of Curitiba being determined by the Laboratório Central do Estado do Paraná (EpiCov GISAID database EPI_ISL_12001963).
Measures of variables
The detection and quantification of the viral material in the samples were carried out using the US-CDC assay for the N1 marker, which detects the presence of copies of the N gene of the SARS-CoV-2, with the RNAse P marker for human ribonuclease being used as a control for reaction inhibition (Lu et al. 2020). Rt-qPCR reactions of 20 µL were performed in a Quantstudio 5 real-time PCR system (Applied Biosystems, Walthan, MA, USA) using primers (reaction final concentration of 0.5 µM/L) and probes (reaction final concentration of 0.125 µM/L) from IDT (Integrated DNA Technologies Inc., Coralville, IA, USA) and the iTaq Universal Probes One-Step kit from Biorad Laboratories (Hercules, CA, USA). All experiments included positive and negative controls. Volumes of reagents and samples used for the reactions are presented in Supplementary Table 2, and qPCR cycling parameters in Supplementary Table 3. As indicated for clinical samples, if the RNAse P control amplified and there was no contamination of the negative controls, reactions that presented a Cq smaller than 40 for the N1 marker were considered positive, and those with a Cq > 40 were deemed to be negative (Lu et al. 2020).
Construction of the standard curve for quantification of the N1 marker was carried out using the IDT 2019-nCoV_N plasmid, with five calibration points in serial dilution 1:10, starting at 20,000 copies µL−1 and ending at 2 copies µL−1. The plasmid concentrations were halved because the plasmids used for the standard curve were double-stranded (ds), but a single-stranded viral RNA was transcribed into dsDNA during the rt-qPCR reaction (Hillary et al. 2021; Mota et al. 2021). Standard curves were performed in all quantification reactions, with 3–5 replicates of each calibration point. In the samples, quantification reactions of the N1 marker were carried out in triplicates. The results were valid when the standard deviation of the Cq between the replicates was lower than 0.5. The 95% confidence interval (CI) for the measures of copies is as:
The RT-qPCR experiments were analyzed using Design & Analysis Software 2.6 (Applied Biosystems). The results were first analyzed using the auto-threshold/baseline function, and if needed, the values of these parameters were adjusted to maximize efficiency during the exponential amplification phase. The calibration curve results were considered acceptable if they fall with the following values: slope -2·97 to -3·63, R2 ≥ 0·99, Y-intercept between 33–37, and efficiency between 90–110%. The limit of detection (LOD) and limit of quantification (LOQ) for the assay was estimated by the methodology of Klymus et al. (2020), which selects the curve fitting method most appropriate for the data using the Akaike Information Criterion, lack of fit and residual variance. The analysis was performed using the script made available by Merkes et al. (2019), with the LOQ coefficient of variation threshold set to 0.35. The best curve-fitting model selected was the second-order polynomial model, with an estimated LOD estimated of 8,03 copies µL−1 for a single replicate, and of 1.34 copies µL−1 for three replicates, and an estimated LOQ of 1,823 copies µL−1. The conversion from copies µL−1 to copies L−1 was performed considering the final elution volume during the RNA extraction (100 µL) and the volume of sample filtered (in mL), as follows:
Converting the LOD for three replicates using the mean filtered volume indicated above, the LOD was of 2,146.37 copies L−1. To obtain the viral load for each WWTP at each sampling day, the measured values of copies per L for a sample was multiplied by the average of the flow (L/s) during the automated sampling period (4 h) and by 24 h to represent a full day.
Concerning the clinical data obtained from the municipality, positive tests have been defined as new daily cases by testing date, while the reported cases as new daily cases by report date. The main difference between these two first indicators is that the number of daily positive tests has been notified weekly and with a delay of five days, while the reported cases are notified daily as they are received by the municipal administration. In addition, the municipal administration defined an active case as a person in the active phase of the disease with potential for transmission, with the total value being given by confirmed cases – [deaths + recovered], with the active phase period being defined as 14 days during 2021, and 10 days from January 2022 onwards. To obtain the daily number of reported cases associated with each WWTP, the reported cases for each city district attended by a given WWTP were summed up, with districts attended by more than one plant being added to both. Information on the waves of the SARS-CoV-2 variants of concern (VOC) Gamma, Delta, and Omicron (lineage BA.1) were obtained from Adamoski et al. (2021, 2022) and from Paraná State Government press releases, with the first register of Omicron lineage BA.2 in the metropolitan region of Curitiba being determined by the Laboratório Central do Estado do Paraná (EpiCov GISAID database EPI_ISL_12001963).
Data analysis procedure
All statistical analyses were performed using R 4.0.3 (R Core Team 2021). Initial exploring of the epidemiological data obtained from the COVID-19 Curitiba Panel for the daily number of positive tests, reported cases, and reported cases by WWTP showed that these data were often undertested and underreported on weekends because of the so-called "weekend effect" (Soukhovolsky et al. 2021). Due to this, before further analysis, these three epidemiological parameters were smoothed using a moving average method with seven adjacent days using the function rollmean of the zoo R package (Zeileis and Grothendieck 2005). The number of active cases did not show a clear "weekend effect" pattern, so it was not smoothed. The values of N1 copies L−1 were used to calculate the daily viral load per sampling point by multiplying the obtained values by the mean flow value (L s−1) times 24 h. To account for missing data of daily viral load (one time in two WWTPs) and the different days of sampling due to holidays (four times) was performed data imputation using a linear interpolation implemented by the function na.interp of the forecast R package (Hyndman et al. 2021). These values were then summed to obtain the total daily viral load.
As viral loads (from each WWTP and total) and the clinical data do not follow a normal distribution (Shapiro–Wilk test, p-values < 0.05, not show), the correlation between variables were tested using the non-parametric Spearman rank correlation coefficient (alpha = 0.05). Weekly viral titers were tested for correlation with daily numbers of each epidemiological indicator, from the day of sampling and for the 20 subsequent days. The correlation was performed using the cor_mat function of the rstatix package (Kassambara 2021), with the p values being estimated with the cor.mtest function of the corrplot R package (Wei and Simko 2021).The correlation between the wastewater viral loads and the number of reported cases were also evaluated using a cross-correlation function (Ccf), which evaluates the displacement of a time series in relation to another, using the CCF function of the feasts R package (O’Hara-Wild et al. 2021), with a maximum of four lag units (four weeks or 28 days). The relation of the total wastewater loads with the different epidemiological indicators available was analyzed by the daily load values obtained for each WWTP summed and tested for correlation as described above with the daily number of positive tests, new cases, and active cases of COVID-19.
Additionally, to analyze if the wastewater loads were similar during the periods with different SARS-CoV-2 VOCs, the data were divided into three waves, Gamma VOC (monitoring weeks 1 to 13), Delta VOC (weeks 14 to 41), and Omicron VOC (weeks 42 to 92). To evaluate if there were any changes in the individual viral loads between these periods, besides comparing the raw daily SARS-CoV-2 wastewater loads, a daily number of viral copies per gram of feces was also calculated. For this, we divided the daily citywide SARS-CoV-2 titers by the number of active cases in the given day divided for the mean value of 149 g grams of individual daily fecal mass (Rose et al. 2015). As the three periods were not evenly sampled, and both variables do not follow a normal distribution (Shapiro–Wilk test, p-value < 0.05, not shown), the significance of the differences in the means between these periods and its 95% confidence interval (95%CI) was accessed through a two-way permutation test with 10,000 replicates. These tests were performed using the permTS function of the perm R package (Fay and Shaw 2010).
Results
As an overview of the dynamics of the wastewater COVID-19 loads in Curitiba during the monitored period (Fig. 2, which started nearly one year after the first case report, is is possible to say that at the beginning of the quantification the pandemic curve was in a wave of new COVID-19 cases, so the peak of virus in the sewage may not have been detected in this period. However, the other 3 peaks of SARS-CoV-2 RNA in the sewage were found as follows: (i) on 25th May 2021, the load of SARS-CoV-2 RNA in the sewage influent to the WWTPs achieved 13 trillion genomic copies per day, resulting in more than a thousand new daily cases of COVID-19 reported in the following days; (ii) on 18th January 2022, 19 trillion of genomic copies per day were detected in sewage influent to the WWTPs, which resulted in more the 4,000 new daily cases of COVID-19 reported nine days later; (iii) on 24th May, 2022, viral load similar to the last peak was observed in sewage, while the reported new daily cases of COVID-19 formed a plateau with more than 1,500 cases; and (iv) on 15th November 2022 there was another surge with values similar to the ones found in May 2021. On the other hand, mid-September to December 2021, late January to the beginning of April 2022, and mid-September to mid-October 2022 were the lighter moments of the COVID-19 pandemic in Curitiba. In these periods, the SARS-CoV-2 RNA load in sewage influent to the WWTPs remained below 833 billion genomic copies per day, and reported cases progressively decreased until they were less than 900 active cases of COVID-19. Most of the samples with no viral detection occurred between October to December 2021 (18 out of a total of 458 samples), although there was also no detection for a single sample from the first week of May 2022 and the last week of September 2022.
Total loads of SARS-CoV-2 in the wastewater (black lines) and 95% confidence interval of the quantification (grey shaded area) in relation to different panels of epidemiological indicators (columns) in Curitiba, Brazil, from March 2021 to November 2022. Background colors indicate the waves of COVID-19 variant of concern (VOC) a. Daily number of positive tests. b. Reported cases. c. Active cases
Correlation between individual wastewater treatment plant SARS-CoV-2 loads and cases by region attended
The viral load in sewage significantly correlated with clinical data obtained for each region from the open database of COVID-19 cases. Results of Ccf analysis by sampling point are show in the Supplementary Information (SI) (SI Table S1) indicate that the viral loads in the WWTP showed moderate to high correlation with the local number of cases in the week following the sampling (lag 1, 7 days after sampling), except for the WWTP Atuba Sul region, which showed moderate Ccf values for lag 0, and WWTP Belém, that presented a higher Ccf value with lag 2 (14 days after sampling) (SI Fig. S1, Table S1). Spearman correlation analysis between the WWTPs viral loads and the number of cases for the districts (SI Table S2) suggests at least a monotonic relationship between the variables, where both tend to move in the same direction but not necessarily at a constant rate as in a linear relationship. The R2 values indicated that the WWTPs showed a low-medium degree of linearity in the correlation between wastewater loads and the number of cases (SI Fig S2), except the WWTP Santa Quitéria region presented a strong correlation. Even so, the discordance between the results of Ccf analysis, adjusted R2, and Spearman correlation values suggest that a monotonic relationship does not adequately describe the pattern of variation between the two measures, which seems to be best captured by using the Ccf. Between WWTPs, the SARS-CoV-2 wastewater loads per 100,000 habitants presented Spearman correlation values ranging from 0.63–72, but only the correlations between three pairs of WWTs were significant (p < 0.05) (SI Table S3).
Evaluation of the performance of different epidemiological indicators in relation to the citywide measures of SARS-CoV-2 loads in wastewater
The results for the combined data showed, by all methods of analysis, a higher correlation to the citywide measures of SARS-CoV-2 loads in wastewater than for the individual measurements in each WWTP (Fig. 2 and SI Figs S1-2; Tables 2 and SI S1-4). Spearman correlation of the total N1 wastewater viral loads showed a high positive correlation with the three epidemiological indicators evaluated (Table SI S4), with the higher correlation values being registered three days after sampling for the number of positive tests (Fig. 2a), eight days for the reported cases (Fig. 2b), and 11 days for the active cases (Fig. 2c). The values of the adjusted R2 for the days with the highest Spearman correlation indicated that the daily number of positive tests and the newly reported cases presented a more linear relationship with the total N1 viral loads (respectively, adjusted R2 0.72 and 0.69) than the one found for the active cases (adjusted R2 0.44). The Ccf analysis returned results similar to the ones found by the Spearman correlation, with the total wastewater N1 viral load presenting the higher correlation with the positive tests at lag 0 (sampling day), at lag 1 (seven days) for the reported cases, and lag 2 (14 days) for the active cases (Table 2).
Effects of different waves of SARS-CoV-2 variants of concern on the results of viral titers in wastewater
Comparing the total SARS-CoV-2 wastewater loads during the periods characterized by the presence of different VOCs waves (Fig. 3a), there were no significant differences between the loads registered in the period of the Gamma VOC wave and the Delta VOC wave (two-way permutation test, p = 0.28, 95%CI 0.27–0.30), or between the waves of the Gamma VOC and the Omicron VOC (two-way permutation test, p = 0.1, 95%CI 0.01–0.11). However, the average values registered after the first detection of the Omicron VOC were about 2.69 times higher than the ones measured after the first detection of the Delta VOC (two-way permutation test, p < 0.01, 95% CI 1 × 10–3-2 × 10–3).
Boxplot of Mean SARS-CoV-2 wastewater loads and viral gene copies per gram of fecal mass in Curitiba, Brazil (from March 2021 to November 2022a.) in the waves of variants of concern (VOC). Each boxplot indicates, for a given wave, the mean (bold line), the quartiles of the data (box), the range (whiskers), and outliers (black dots). a. Mean total SARS-CoV-2 wastewater loads. b. SARS-CoV-2 N1 gene copies per gram of fecal mass
Considering the day with the highest correlation value between the daily viral load and the number of active cases (11 days after sampling), measured as number of gene copies per gram of fecal mass (Fig. 3b), there were no significant differences between the numbers of gene copies per gram of fecal mass during the Gamma VOC wave in relation to the Delta VOC wave (two-way permutation test, p = 0.59, 95%CI 0.58–0.60). Even so, the values obtained for these first two variants were significantly smaller than the one found during the Omicron VOC wave (two-way permutation test, respectively, Omicron and Gamma, p < 0.01, 95%CI 5 × 10–3-0.01, and Omicron and Delta, p < 0.01, 95%CI 1 × 10–5-5 × 10–4), with the virus fecal load increasing between 5–6 times after the introduction of this last VOC.
Even after being separated by the VOC periods, the total SARS-CoV-2 wastewater loads and the number of gene copies per gram of fecal mass showed the same pattern in relation to the three epidemiological indicators as the data for the whole period (SI Table S5), with higher values of Spearman correlation with the number of positive tests than with the other indicators (Table S5), and with positive cases presenting a higher ccf correlation at lag 0 (sampling day) (SI Table S6). The ccf with reported cases (Table S6) was higher at lag 0 for the Gamma and Delta periods and at lag 1 (seven days after sampling) in the Omicron period. Considering the ccf with active cases (Table S6), for the Gamma period it was higher at lag 3 (21 days after sampling), for the Delta period higher at lag 0 (sampling day), and higher at lag 2 (14 days after sampling) for the Omicron period.
Discussion
In summary, our findings showed the existence of local variations in the SARS-CoV-2 wastewater loads across different areas of Curitiba city, that the total measured viral titers presented the smallest lag or response time in relation to the epidemiological indicator of a daily number of positive tests, and that the waves of distinct VOCs lead to differences in the measure wastewater loads.
Regional differences between SARS-CoV-2 wastewater loads influent on the WWTPs and clinical data are expected due to local dynamics of COVID-19 infection and seem reflected in the slightly different lags or response times found for each WWTP analyzed. Hoar et al. (2022) also reported a similar scenario for New York City, and suggested these kinds of differences might also be attributed to particularities in the sewer networks, including residence times, non-domestic water inputs, the proportion of transient individuals, and per capita water use, as well as to differences in clinical testing rates. In Curitiba, in addition to these factors, the relationship between the wastewater viral loads and the regionalized number of reported cases found for the WWTPs seems to be related to the area and size of the population attended by each WWTP. It can be observed that the smaller WWTPs (CIC-Xisto, Padilha Sul, and Santa Quitéria), presented its highest correlation values with lag 1 (seven days after sampling), whereas the largest WTTPs (Atuba and Belém) showed higher correlation, respectively, with lag -1 (seven days before sampling) or lag 2 (14 days after sampling). A systematic review of correlation measurements of viral titers in sewage and the number of COVID-19 cases reported values ranging -0.38 to 0.99 (Li et al. 2023b), with studies in Brazil showing positive correlations between 0.41–0.63 (Bueno et al. 2022), and regression coefficients ranging from 0.22–0.71 (Barbosa et al. 2022; Razzolini et al. 2021). In comparison to the values reported for Brazil, the ones found for Curitiba in the present study are among the highest found for the country, which can be related to the high sewer coverage in the city. It is important to note that, probably due to variations in data collection and analysis associated with inappropriate testing and human-to-human shedding variation, there is no clear correlation among SARS-CoV-2 wastewater loads and active cases found in different countries (Parida et al. 2023).
Monitoring epidemics in real-time still presents numerous challenges, including reporting delays caused by factors such as laboratory confirmations, logistical hurdles, infrastructure limitations, and other related issues (Bastos et al. 2019). These challenges have also impacted the effectiveness of measures implemented during the COVID-19 pandemic (Sarnaglia et al. 2022). Our results showed that the lag between the viral measurements and the number of positive tests is higher for the sampling week, similar to the found by Martins et al. (2022) in São José do Rio Preto (Brazil) for positive clinical cases, and for positive tests in two localities of Ontario, Canada (Hegazy et al. 2022). As found by Li et al. (2023a, b), our analysis found higher correlation measures for positive tests and daily reported cases than for active cases, and besides shows that different epidemiological indicators can have a relevant impact in its response time to be used as an early warning system. Such delays can be related to the lag of about one week between positive tests and reported cases and the wastewater viral loads, or the even larger lag between the loads and the number of active cases, which possibly were also affected by the change in the definition of active cases used by Curitiba municipal administration. As, in general, a positive test preceded the reporting of a given case or its consideration as an active one, dates from positive tests allow that the time to develop COVID-19 after contracting the virus ranges between 1 to 14 days, with a mean incubation period of 5–6 days (Li et al. 2020; Linton et al. 2020; Guan et al. 2020; Lauer et al. 2020), and although the virus can be detected in the upper respiratory tract 1 to 3 days before the onset of the symptoms (WHO 2020), initial recommendations for sample collection to testing were approximately 8 days after the onset of symptoms (Lima et al. 2021). According to Kucirka et al. (2020), the probability of an infected person's false-negative result of an RT-PCR-based test is 38% on the day of the first symptom. This value decreases to 20% on the third day after the symptom onset. Thus, COVID-19 testing has been recommended 2–10 days after the first symptoms for an etiological diagnosis (da Rocha Araujo 2020), and in Curitiba, the municipal administration recommends testing between 3 to 7 days after the onset of the symptoms.
Although very important in clinical and health settings, these directives about when a test is recommended can lead to another small delay in discovering an outbreak of the disease. On the other hand, gastrointestinal manifestations of COVID-19 can appear on average 4.9 days before hospital admission (Buscarini et al. 2020), with stool testing for the disease showing positive results since the onset of the symptoms (Britton et al. 2021). Then, it is possible that viral shedding in feces occurs 3–5 days before other classic symptoms (Jones et al. 2020). Given these considerations, SARS-CoV-2 wastewater loads can offer a near real-time indicator of the number of positive tests. According to findings, it has the ability to detect changes in the infection dynamics even up to four days prior to individual testing, making it a trustworthy proxy for the number of infected in the days following sampling. Additionally, clinical testing alone frequently fails to detect presymptomatic, asymptomatic, and mild symptomatic cases, which also contribute to the spread of COVID-19, with data exclusive from diagnostics representing inadequate surveillance data to decision making by public health agents (Kumblathan et al. 2023).
The viral loads found in the waves of different COVID-19 VOCs suggest that the viral lineages in circulation can strongly affect the quantification of the virus in the sewage. Some reports demonstrated that in clinical testing the VOCs Delta, and Omicron variants produce higher viral titers than the Gamma VOC (Fall et al. 2022; King et al. 2022), and our data suggests that at least the Omicron VOC can be associated with higher SARS-CoV-2 RNA loads in sewage that the Delta VOC, although the results do not support the same in an increase in the comparison between Delta and Gamma VOCs. The values of gene copies per gram of fecal mass calculated from the wastewater SARS-CoV-2 viral titers and the number of active cases for Curitiba mainly fall within values described in the literature, ranging between 4.3 × 103 to 1.3 × 108 gene copies g−1 (Kitajima et al. 2020; Fumian et al. 2021), but the values found for the period with a predominance of the Omicron VOC were significantly higher than the ones found for the others VOCs. Hegazy et al. (2022) showed that for Ontario (Canada) the correlation and lag times were affected by periods with a predominance of the Alpha VOC, Delta VOC, and higher vaccination levels, and the surge of the Omicron VOC, but the analysis of our data for the waves of the different variants showed a little variation on the correlation values (smaller for the Gamma VOC) and no variation on the lag times, with all VOCs showing higher values at lag 0 (sampling day) for the positive tests indicator (note that indicators “reported cases”and “active cases”showed variation for correlation and lag times). As both the raw SARS-CoV-2 wastewater titers (Fig. 3a) and the viral load by the case (Fig. 3b, expressed in copies per gram of fecal mass) showed some degree of change between the different VOC waves, it is not clear if the observed changes in the viral titer patterns are due to the generation of higher SARS-CoV-2 loads in the infected individuals by the Omicron VOC or due to different rates of transmission between the VOCs. Even so, the results presented showed that, in comparison with the best-fit epidemiological indicator, SARS-CoV-2 monitoring in wastewaters could deliver robust results regarding the infection dynamics in a population.
Conclusion, limitations and prospects
The results of this study represent one more piece of evidence of the utility of SARS-CoV-2 wastewater monitoring as an early warning system, which can provide robust results even during different viral variant waves if analyzed in relation to a more precise epidemiological indicator as the number of positive tests. Thus, based on the analysis performed herein we recommend the use of a daily number of positive tests as a more efficient epidemiological indicator, which seems not to be affected by the changes in the waves of SARS-CoV-2 VOCs. However, is worth noting that the changes in the definition of active cases by the municipality from 2022 onwards could have affected the results regarding this indicator. Likewise, regarding the waves of COVID-19 VOCs, the differences in the viral loads per case during the the Omicron VOC wave may have been boosted by the liberation of self-testing (with no compulsory notification) by the Brazilian government at the end of January 2022..Even with these limitations, and considering that there are still several challenges in the wastewater based epidemiology approach to be solved regarding standardized methodologies, sewer variation, and data analysis (Ciannella et al. 2023; Jiang et al. 2023), this approach was demonstrated to be robust to deal intrinsic variations in the data.
More, the SARS-CoV-2 virus can be found in several environmental matrices, including aerosols and different kinds of solid surfaces (Vicente et al. 2021; Parida et al. 2023), with its presence in natural and human-made water bodies (including wastewater) derived mainly from stool and urine (Núñez-Delgado et al. 2021; Parida et al. 2023). It is worth noting that, despite the low number of publications on the persistence of viable SARS-CoV-2 in excreta or sewage (Wölfel et al. 2020; Xiao et al. 2020; Jeong et al. 2020; Fumian et al. 2021; Wurtzer et al. 2021) the World Health Organization still considers the potential transmission of COVID-19 through wastewater as unconfirmed, and due to precaution principles, it is highly recommended the use of collective and personal protective equipment by WWTPs workers and that treatment tanks are covered (WHO 2020; Zaneti et al. 2021). On other hand, although viral presence in wastewater allows for very effective tracking and quantification of the infected individuals in a population, it does not seems to represent a new route of transmission or an important factor in determining the dynamics of viral infections in a community when compared with air pollution or meteorological conditions (Coccia 2020; Barcellos et al. 2021; Mathys et al. 2023).
The COVID-19 pandemic showed that there is a lot to learn and to improve in our ability to face the fast spread of a pathogenic agent and manage future crisis scenarios (Coccia 2021). Vaccination was the main pharmaceutical measure that leads to a reduction in the number of infected individuals and lethal cases (Coccia 2022b, c), and it has been shown that good governance can increase vaccination levels (Benati and Coccia 2022a) Among non-pharmaceutical measures for crisis management, aside from more effective new technologies (Coccia 2023), the most common social policies adopted where lockdowns, which were effective but restricted the population mobility (Alfano and Ercolano 2020; Cai et al. 2021; Secco and Conte 2022) and contact tracing (Benati and Coccia 2022b), which were less widely adopted in countries with limited resources as Brazil. It has been argued that the adoption of more comprehensive prevention strategies in the case of new epidemics in the future must also be based on sustainability and environmental sciences (Coccia 2020).
To conclude, WBE approaches that have already been traditionally used for drug use surveillance (Luo et al. 2023), proved in the COVID-19 pandemic that can represent essential pieces of an integrated prevention approach, providing precise data and almost real-time data and at a lower cost than individual testing. The future development of standardized methods (Jiang et al. 2023), risk index approaches to inform decision makers (Asadi et al. 2023), and data-driven methods to predict variables related to outbreaks (Ciannella et al. 2023) will allow the WBE approach to be more widely utilized in comparative studies and for decision-making policies.
Data availability
The epidemiological data, wastewater SARS-COV-2 quantification data, and the R code used in for the analysis are available at https://doi.org/10.6084/m9.figshare.22149359.v2
References
Adamoski D, de Oliveira JC, Bonatto AC et al (2021) Large-scale screening of asymptomatic persons for SARS-CoV-2 variants of concern and gamma takeover, Brazil. Emerg Infect Dis 27:3124–3127. https://doi.org/10.3201/eid2712.211326
Adamoski D, de Baura VA, Rodrigues AC et al (2022) SARS-CoV-2 delta and omicron variants surge in Curitiba, Southern Brazil, and its impact on overall COVID-19 lethality. Viruses 14:809. https://doi.org/10.3390/v14040809
Agrawal S, Orschler L, Lackner S (2021a) Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Sci Rep 11:5372. https://doi.org/10.1038/s41598-021-84914-2
Agrawal S, Orschler L, Schubert S, et al (2021b) A pan-European study of SARS-CoV-2 variants in wastewater under the EU Sewage Sentinel System. medRxiv 6(11):21258756. https://doi.org/10.1101/2021.06.11.21258756
Ahmed W, Harwood VJ, Gyawali P et al (2015) Comparison of concentration methods for quantitative detection of sewage-associated viral markers in environmental waters. Appl Environ Microbiol 81:2042–2049. https://doi.org/10.1128/AEM.03851-14
Ahmed W, Angel N, Edson J et al (2020) First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ 728:138764. https://doi.org/10.1016/j.scitotenv.2020.138764
Alfano V, Ercolano S (2020) The efficacy of lockdown against COVID-19: a cross-country panel analysis. Appl Health Econ Health Policy 18:509–517. https://doi.org/10.1007/s40258-020-00596-3
Arora S, Nag A, Sethi J et al (2020) Sewage surveillance for the presence of SARS-CoV-2 genome as a useful Wastewater Based Epidemiology (WBE) tracking tool in India. Water Sci Technol 82:2823–2836. https://doi.org/10.2166/wst.2020.540
Asadi M, Oloye FF, Xie Y et al (2023) A wastewater-based risk index for SARS-CoV-2 infections among three cities on the Canadian Prairie. Sci Total Environ 876:162800. https://doi.org/10.1016/j.scitotenv.2023.162800
Baj J, Karakuła-Juchnowicz H, Teresiński G et al (2020) COVID-19: Specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med 9:1–22. https://doi.org/10.3390/jcm9061753
Barbosa MRF, Garcia SC, de Castro Bruni A et al (2022) One-year surveillance of SARS-CoV-2 in wastewater from vulnerable urban communities in metropolitan São Paulo, Brazil. J Water Health 20:471–490. https://doi.org/10.2166/wh.2022.210
Bar-Or I, Weil M, Indenbaum V et al (2021) Detection of SARS-CoV-2 variants by genomic analysis of wastewater samples in Israel. Sci Total Environ 789:148002. https://doi.org/10.1016/j.scitotenv.2021.148002
Bastos LS, Economou T, Gomes MFC et al (2019) A modelling approach for correcting reporting delays in disease surveillance data. Stat Med 38:4363–4377. https://doi.org/10.1002/sim.8303
Benati I, Coccia M (2022a) Global analysis of timely COVID-19 vaccinations: improving governance to reinforce response policies for pandemic crises. Int J Health Gov 27:240–253. https://doi.org/10.1108/IJHG-07-2021-0072
Benati I, Coccia M (2022b) Effective contact tracing system minimizes COVID-19 related infections and deaths: policy lessons to reduce the impact of future pandemic diseases. J Public Adm Gov 12:19–33. https://doi.org/10.5296/jpag.v12i3.19834
Britton GJ, Chen-Liaw A, Cossarini F et al (2021) Limited intestinal inflammation despite diarrhea, fecal viral RNA and SARS-CoV-2-specific IgA in patients with acute COVID-19. Sci Rep 11:13308. https://doi.org/10.1038/s41598-021-92740-9
Buscarini E, Manfredi G, Brambilla G et al (2020) GI symptoms as early signs of COVID-19 in hospitalised Italian patients. Gut 69:1547–1548. https://doi.org/10.1136/gutjnl-2020-321434
Cai M, Guy C, Héroux M et al (2021) The impact of successive COVID-19 lockdowns on people mobility, lockdown efficiency, and municipal solid waste. Environ Chem Lett 19:3959–3965. https://doi.org/10.1007/s10311-021-01290-z
Carrillo-Reyes J, Barragán-Trinidad M, Buitrón G (2021) Surveillance of SARS-CoV-2 in sewage and wastewater treatment plants in Mexico. J Water Process Eng 40:101815. https://doi.org/10.1016/j.jwpe.2020.101815
Chernicharo CAL, Araújo JC, Mota Filho CR et al (2020) Monitoramento do esgoto como ferramenta de vigilância epidemiológica para controle da Covid-19: Estudo de caso na cidade de Belo Horizonte. Eng Sanit Ambient 26:691–699. https://doi.org/10.1590/S1413-415220200243
Ciannella S, González-Fernández C, Gomez-Pastora J (2023) Recent progress on wastewater-based epidemiology for COVID-19 surveillance: a systematic review of analytical procedures and epidemiological modeling. Sci Total Environ 878:162953. https://doi.org/10.1016/j.scitotenv.2023.162953
Claro ICM, Cabral AD, Augusto MR et al (2021) Long-term monitoring of SARS-CoV-2 RNA in wastewater in Brazil: a more responsive and economical approach. Water Res 203:117534. https://doi.org/10.1016/j.watres.2021.117534
Coccia M (2020) Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci Total Environ 729:138474. https://doi.org/10.1016/j.scitotenv.2020.138474
Coccia M (2021) Pandemic prevention: lessons from COVID-19. Encyclopedia 1:433–444. https://doi.org/10.3390/encyclopedia1020036
Coccia M (2022a) Preparedness of countries to face COVID-19 pandemic crisis: strategic positioning and factors supporting effective strategies of prevention of pandemic threats. Environ Res 203:111678. https://doi.org/10.1016/j.envres.2021.111678
Coccia M (2022b) Optimal levels of vaccination to reduce COVID-19 infected individuals and deaths: a global analysis. Environ Res 204:112314. https://doi.org/10.1016/j.envres.2021.112314
Coccia M (2022c) Improving preparedness for next pandemics: max level of COVID-19 vaccinations without social impositions to design effective health policy and avoid flawed democracies. Environ Res 213:113566. https://doi.org/10.1016/j.envres.2022.113566
Coccia M (2023) High potential of technology to face new respiratory viruses: mechanical ventilation devices for effective healthcare to next pandemic emergencies. Technol Soc 73:102233. https://doi.org/10.1016/j.techsoc.2023.102233
da Rocha Araujo FAG (2020) Uses and limits of the clinical laboratory in the COVID-19 pandemic: a didactic review. Rev Assoc Med Bras 66:1718–1724. https://doi.org/10.1590/1806-9282.66.12.1718
da Rosa Mesquita R, Silva Junior LCF, Santana FMS et al (2021) Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr 133:377–382. https://doi.org/10.1007/s00508-020-01760-4
da Silveira Barcellos D, Fernandes GMK, de Souza FT (2021) Data based model for predicting COVID-19 morbidity and mortality in metropolis. Sci Rep 11:24491. https://doi.org/10.1038/s41598-021-04029-6
de Freitas Bueno R, Claro ICM, Augusto MR et al (2022) Wastewater-based epidemiology: a Brazilian SARS-CoV-2 surveillance experience. J Environ Chem Eng 10:108298. https://doi.org/10.1016/j.jece.2022.108298
Fall A, Eldesouki RE, Sachithanandham J et al (2022) The displacement of the ovariant Delta with Omicron: an investigation of hospital admissions and upper respiratory viral loads. eBioMedicine 79:104008. https://doi.org/10.1016/j.ebiom.2022.104008
Fay MP, Shaw PA (2010) Exact and asymptotic weighted logrank tests for interval censored data: the interval R package. J Stat Softw 36:1–34. https://doi.org/10.18637/jss.v036.i02
Feng S, Owens SM, Shrestha A et al (2023) Intensity of sample processing methods impacts wastewater SARS-CoV-2 whole genome amplicon sequencing outcomes. Sci Total Environ 876:162572. https://doi.org/10.1016/j.scitotenv.2023.162572
Fongaro G, Rogovski P, Savi BP et al (2022) SARS-CoV-2 in Human sewage and river water from a remote and vulnerable area as a surveillance tool in Brazil. Food Environ Virol 14:417–420. https://doi.org/10.1007/s12560-021-09487-9
Fumian TM, Malta FC, dos Santos DRL et al (2021) SARS-CoV-2 RNA detection in stool samples from acute gastroenteritis cases, Brazil. J Med Virol 93:2543–2547. https://doi.org/10.1002/jmv.26786
Gerrity D, Papp K, Stoker M et al (2021) Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: methodology, occurrence, and incidence/prevalence considerations. Water Res X 10:100086. https://doi.org/10.1016/j.wroa.2020.100086
Giraud-Billoud M, Cuervo P, Altamirano JC et al (2021) Monitoring of SARS-CoV-2 RNA in wastewater as an epidemiological surveillance tool in Mendoza, Argentina. Sci Total Environ 796:148887. https://doi.org/10.1016/j.scitotenv.2021.148887
Gonzalez R, Curtis K, Bivins A et al (2020) COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res 186:116296. https://doi.org/10.1016/j.watres.2020.116296
Guan W, Ni Z, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1056/NEJMoa2002032
Hasan SW, Ibrahim Y, Daou M et al (2021) Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: surveillance of COVID-19 epidemic in the United Arab Emirates. Sci Total Environ 764:142929. https://doi.org/10.1016/j.scitotenv.2020.142929
Hegazy N, Cowan A, D’Aoust PM et al (2022) Understanding the dynamic relation between wastewater SARS-CoV-2 signal and clinical metrics throughout the pandemic. Sci Total Environ 853:158458. https://doi.org/10.1016/j.scitotenv.2022.158458
Hillary LS, Farkas K, Maher KH et al (2021) Monitoring SARS-CoV-2 in municipal wastewater to evaluate the success of lockdown measures for controlling COVID-19 in the UK. Water Res 200:117214. https://doi.org/10.1016/j.watres.2021.117214
Hoar C, Chauvin F, Clare A et al (2022) Monitoring SARS-CoV-2 in wastewater during New York City’s second wave of COVID-19: sewershed-level trends and relationships to publicly available clinical testing data. Environ Sci Water Res Technol 8:1021–1035. https://doi.org/10.1039/D1EW00747E
Hyndman RJ, Athanasopoulos G, Bergmeir C (2021) Forecasting functions for time series and linear models. R package. version 8.14. https://cran.r-project.org/web/packages/forecast. Accessed in 15 May 2023.
Jeong HW, Kim S-M, Kim H-S et al (2020) Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin Microbiol Infect 26:1520–1524. https://doi.org/10.1016/j.cmi.2020.07.020
Jiang G, Liu Y, Tang S et al (2023) Moving forward with COVID-19: Future research prospects of wastewater-based epidemiology methodologies and applications. Curr Opin Environ Sci Health 33:100458. https://doi.org/10.1016/j.coesh.2023.100458
Johansson MA, Quandelacy TM, Kada S et al (2021) SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open 4:e2035057. https://doi.org/10.1001/jamanetworkopen.2020.35057
Jones DL, Baluja MQ, Graham DW et al (2020) Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci Total Environ 749:141364. https://doi.org/10.1016/j.scitotenv.2020.141364
Karthikeyan S, Ronquillo N, Belda-Ferre P et al (2021) High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSystems 6:1–6. https://doi.org/10.1128/mSystems.00045-21
Kassambara A (2021) rstatix: Pipe-friendly framework for basic statistical tests. R package. version 0.7.0. https://cran.r-project.org/web/packages/rstatix. Accessed in 15 May 2023
King KL, Wilson S, Napolitano JM et al (2022) SARS-CoV-2 variants of concern Alpha and Delta show increased viral load in saliva. PLoS One 17:e0267750. https://doi.org/10.1371/journal.pone.0267750
Kitajima M, Ahmed W, Bibby K et al (2020) SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci Total Environ 739:139076. https://doi.org/10.1016/j.scitotenv.2020.139076
Klymus KE, Merkes CM, Allison MJ et al (2020) Reporting the limits of detection and quantification for environmental DNA assays. Environ DNA 2:271–282. https://doi.org/10.1002/edn3.29
Krivoňáková N, Šoltýsová A, Tamáš M et al (2021) Mathematical modeling based on RT-qPCR analysis of SARS-CoV-2 in wastewater as a tool for epidemiology. Sci Rep 11:1–10. https://doi.org/10.1038/s41598-021-98653-x
Kucirka LM, Lauer SA, Laeyendecker O et al (2020) Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 173:262–267. https://doi.org/10.7326/M20-1495
Kumblathan T, Liu Y, Qiu Y et al (2023) An efficient method to enhance recovery and detection of SARS-CoV-2 RNA in wastewater. J Environ Sci 130:139–148. https://doi.org/10.1016/j.jes.2022.10.006
Lauer SA, Grantz KH, Bi Q et al (2020) The incubation period of Coronavirus Disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 172:577–582. https://doi.org/10.7326/M20-0504
Le C (2023) Sensitivity of wastewater surveillance: what is the minimum COVID-19 cases required in population for SARS-CoV-2 RNA to be detected in wastewater? J Environ Sci 125:851–853. https://doi.org/10.1016/j.jes.2022.08.020
Li Q, Guan X, Wu P et al (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. https://doi.org/10.1056/nejmoa2001316
Li Q, Lee BE, Gao T et al (2023a) Number of COVID-19 cases required in a population to detect SARS-CoV-2 RNA in wastewater in the province of Alberta, Canada: sensitivity assessment. J Environ Sci 125:843–850. https://doi.org/10.1016/j.jes.2022.04.047
Li X, Zhang S, Sherchan S et al (2023b) Correlation between SARS-CoV-2 RNA concentration in wastewater and COVID-19 cases in community: a systematic review and meta-analysis. J Hazard Mater 441:129848. https://doi.org/10.1016/j.jhazmat.2022.129848
Lima FET, de Albuquerque NLS, de Souza Gurgel Florencio S et al (2021) Intervalo de tempo decorrido entre o início dos sintomas e a realização do exame para COVID-19 nas capitais brasileiras, agosto de 2020*. Epidemiol e Serviços Saúde 30:e2020788. https://doi.org/10.1590/s1679-4974202100010002
Linton N, Kobayashi T, Yang Y et al (2020) Incubation period and other epidemiological characteristics of 2019 Novel Coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med 9:538. https://doi.org/10.3390/jcm9020538
Lu X, Wang L, Sakthivel SK et al (2020) US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis 26:1654–1665. https://doi.org/10.3201/eid2608.201246
Luo J, Bello D, Pagsuyoin S (2023) Long-term wastewater-based surveillance and impacts of the COVID-19 pandemic on drug use trends in a U.S. Northeast rural town. Sci Total Environ 877:162806. https://doi.org/10.1016/j.scitotenv.2023.162806
Magazzino C, Mele M, Coccia M (2022) A machine learning algorithm to analyse the effects of vaccination on COVID-19 mortality. Epidemiol Infect 150:e168. https://doi.org/10.1017/S0950268822001418
Mao K, Zhang K, Du W et al (2020) The potential of wastewater-based epidemiology as surveillance and early warning of infectious disease outbreaks. Curr Opin Environ Sci Health 17:1–7. https://doi.org/10.1016/j.coesh.2020.04.006
Martins RM, Carvalho T, Bittar C et al (2022) Long-term wastewater Surveillance for SARS-CoV-2: one-Year Study in Brazil. Viruses 14:2333. https://doi.org/10.3390/v14112333
Mathys T, de Souza FT, da Barcellos D, S, Molderez I, (2023) The relationship among air pollution, meteorological factors and COVID-19 in the Brussels Capital Region. Sci Total Environ 857:158933. https://doi.org/10.1016/j.scitotenv.2022.158933
Medema G, Heijnen L, Elsinga G et al (2020) Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ Sci Technol Lett 7:511–516. https://doi.org/10.1021/acs.estlett.0c00357
Merkes C, Klymus K, Allison M, et al (2019) Reporting the limits of detection (LOD) and quantification (LOQ) for environmental DNA assays: Data. US Geol Surv data release
Mota CR, Bressani-Ribeiro T, Araújo JC et al (2021) Assessing spatial distribution of COVID-19 prevalence in Brazil using decentralised sewage monitoring. Water Res 202:117388. https://doi.org/10.1016/j.watres.2021.117388
Núñez-Delgado A, Bontempi E, Coccia M et al (2021) SARS-CoV-2 and other pathogenic microorganisms in the environment. Environ Res 201:111606. https://doi.org/10.1016/j.envres.2021.111606
O’Hara-Wild M, Hyndman R, Wang E (2021) feasts: feature extraction and statistics for time series. R package. version 0.2.2. https://cran.r-project.org/web/packages/feasts/. Accessed in 15 May 2023
Parida VK, Saidulu D, Bhatnagar A et al (2023) A critical assessment of SARS-CoV-2 in aqueous environment: existence, detection, survival, wastewater-based surveillance, inactivation methods, and effective management of COVID-19. Chemosphere 327:138503. https://doi.org/10.1016/j.chemosphere.2023.138503
Pilevar M, Kim KT, Lee WH (2021) Recent advances in biosensors for detecting viruses in water and wastewater. J Hazard Mater 410:124656. https://doi.org/10.1016/j.jhazmat.2020.124656
Prado T, Fumian TM, Mannarino CF et al (2020) Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro. Brazil 115:115–117. https://doi.org/10.1590/0074-02760200196
Prado T, Fumian TM, Mannarino CF et al (2021) Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res 191:116810. https://doi.org/10.1016/j.watres.2021.116810
R Core Team (2021) R: a language and environment for statistical computing. R foundation for statistical computing. https://www.R-project.org. Accessed in 15 May 2023
Razzolini MTP, Barbosa MRF, de Araújo RS et al (2021) SARS-CoV-2 in a stream running through an underprivileged, underserved, urban settlement in São Paulo, Brazil: a 7-month follow-up. Environ Pollut 290:118003. https://doi.org/10.1016/j.envpol.2021.118003
Rice J, Kannan AM, Castrignanò E et al (2020) Wastewater-based epidemiology combined with local prescription analysis as a tool for temporalmonitoring of drugs trends - A UK perspective. Sci Total Environ 735:139433. https://doi.org/10.1016/j.scitotenv.2020.139433
Rose C, Parker A, Jefferson B, Cartmell E (2015) The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol 45:1827–1879. https://doi.org/10.1080/10643389.2014.1000761
Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433. https://doi.org/10.1016/j.jaut.2020.102433
Salath M, Althaus CL, Neher R et al (2020) COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly 150:4–6. https://doi.org/10.4414/smw.2020.20225
Sarnaglia AJQ, Zamprogno B, Fajardo Molinares FA et al (2022) Correcting notification delay and forecasting of COVID-19 data. J Math Anal Appl 514:125202. https://doi.org/10.1016/j.jmaa.2021.125202
Saththasivam J, El-Malah SS, Gomez TA et al (2021) COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci Total Environ 774:145608. https://doi.org/10.1016/j.scitotenv.2021.145608
Secco EL, Conte S (2022) To lockdown or not to lockdown: analysis of the EU lockdown performance vs. COVID-19 outbreak. Front Med Technol 4:981620. https://doi.org/10.3389/fmedt.2022.981620
Singhal T (2020) A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr 87:281–286. https://doi.org/10.1007/s12098-020-03263-6
SNIS - Brazilian National Sanitation Information (2020) Painel Indicadores: esgoto. In: Ministério do Desenvolv. Reg
Soukhovolsky V, Kovalev A, Pitt A et al (2021) The cyclicity of coronavirus cases: “Waves” and the “weekend effect.” Chaos, Solitons Fractals 144:110718. https://doi.org/10.1016/j.chaos.2021.110718
Street R, Malema S, Mahlangeni N, Mathee A (2020) Wastewater surveillance for Covid-19: an African perspective. Sci Total Environ 743:140719. https://doi.org/10.1016/j.scitotenv.2020.140719
Symonds EM, Verbyla ME, Lukasik JO et al (2014) A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Res 65:257–270. https://doi.org/10.1016/j.watres.2014.07.032
Torabi S, Amirsoleimani A, Dehghan Banadaki M et al (2023) Stabilization of SARS-CoV-2 RNA in wastewater via rapid RNA extraction. Sci Total Environ 878:162992. https://doi.org/10.1016/j.scitotenv.2023.162992
Vicente VA, Lustosa BPR, Grisolia ME, et al (2021) Environmental detection of SARS-CoV-2 virus rna in health facilities in brazil and a systematic review on contamination sources. Int J Environ Res Public Health 18:3824. https://doi.org/10.3390/ijerph18073824
Wei T, Simko V (2021) R package “corrplot”: visualization of a correlation matrix. version 0.92. https://cran.r-project.org/web/packages/corrplot. Accessed in 15 May 2023
Weidhaas J, Aanderud ZT, Roper DK et al (2021) Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci Total Environ 775:145790. https://doi.org/10.1016/j.scitotenv.2021.145790
WHO (2020) Status of environmental surveillance for SARS-CoV-2 virus. https://www.who.int/publications/i/item/WHO-2019-nCoV-sci-brief-environmentalSampling-2020-1. Accessed in 15 May 2023
Wölfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. https://doi.org/10.1038/s41586-020-2196-x
Wu F, Xiao A, Zhang J et al (2021) Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res 202:117400. https://doi.org/10.1016/j.watres.2021.117400
Wurtzer S, Waldman P, Ferrier-Rembert A et al (2021) Several forms of SARS-CoV-2 RNA can be detected in wastewaters: implication for wastewater-based epidemiology and risk assessment. Water Res 198:117183. https://doi.org/10.1016/j.watres.2021.117183
Xiao F, Tang M, Zheng X et al (2020) Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158:1831-1833.e3. https://doi.org/10.1053/j.gastro.2020.02.055
Yaniv K, Shagan M, Lewis YE et al (2021) City-level SARS-CoV-2 sewage surveillance. Chemosphere 283:131194. https://doi.org/10.1016/j.chemosphere.2021.131194
Zaneti RN, Girardi V, Spilki FR et al (2021) Quantitative microbial risk assessment of SARS-CoV-2 for workers in wastewater treatment plants. Sci Total Environ 754:142163. https://doi.org/10.1016/j.scitotenv.2020.142163
Zeileis A, Grothendieck G (2005) zoo : S3 infrastructure for regular and irregular time series. J Stat Softw 14:1–27. https://doi.org/10.18637/jss.v014.i06
Acknowledgements
The authors acknowledge the financial support obtained from the following institutions: Agência Nacional de Água e Saneamento Básico – ANA; Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq; Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES; Instituto Nacional de Ciência e Tecnologia em Estações de Tratamento de Esgoto Sustentáveis – INCT ETEs Sustentáveis (INCT Sustainable Sewage Treatment Plants). Also, we are grateful to Sanepar (Diretoria de Operações), for the involvement and participation of the team of Research and Innovation Management (GPIN) and Sewage Treatment Management (GTESG) and to the Afonso Pena Airport team of professionals who assisted in the research. Finally, we are grateful for the support of the Municipal Health Secretary of Curitiba (SMS).
Funding
This study received financial support from the following institutions: Agência Nacional de Água e Saneamento Básico – ANA; Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq; Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES; Instituto Nacional de Ciência e Tecnologia em Estações Sustentáveis de Tratamento de Esgoto – INCT ETEs Sustentáveis (INCT Sustainable Sewage Treatment Plants). The funder of the study reviewed the study design but had no role in data collection, data analysis, data interpretation, or the drafting of the manuscript.
Author information
Authors and Affiliations
Contributions
Bárbara Z Leite, Cesar R Mota Filho, Emanuel M de Souza, Gustavo RC Possetti, Ramiro G Etchepare, and Vania A Vicente contributed to the study conception and design. Material preparation, data collection and analysis were performed by Bárbara Z Leite, Carlos ER Barquilha, Caroline Kozak, Demian S Barcellos,, Edy HRA Pereira, Fernanda JO Gomes da Costa, Pâmela E Oliveira, Ricardo Belmonte-Lopes and Willian L Martins, and. The first draft of the manuscript was written by Ricardo Belmonte-Lopes, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
CRediT taxonomy
Ricardo Belmonte-Lopes: Methodology, Software, Formal analysis, Investigation, Data Curation, Writing-Original Draft, Writing-Review & Editing,Visualization Carlos ER Barquilha: Software, Formal analysis, Investigation, Resources, Data Curation, Writing-Original Draft, Writing-Review & Editing, Visualization, Validation Caroline Kozak: Methodology, Investigation, Resources, Data Curation, Writing-Review & Editing Demian S Barcellos: Investigation, Data Curation, Writing-Review & Editing, Resources Bárbara Z Leite: Methodology, Resources, Data Curation, Writing-Review & Editing Fernanda JO Gomes da Costa: Resources, Data Curation, Writing-Review & Editing William L Martins: Investigation, Data Curation Pâmela E Oliveira: Investigation, Data Curation Edy HRA Pereira: Investigation, Resources, Data Curation Cesar R Mota Filho: Conceptualization, Methodology, Resources, Writing-Review & Editing, Supervision, Project administration, Funding acquisition Emanuel M de Souza: Resources, Writing-Review & Editing Gustavo RC Possetti: Conceptualization, Methodology, Resources, Writing-Review & Editing, Project administration Vania A Vicente: Conceptualization, Methodology, Resources, Writing-Review & Editing, Supervision, Project administration Ramiro G Etchepare: Conceptualization, Methodology, Resources, Writing-Review & Editing, Supervision, Project administration
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belmonte-Lopes, R., Barquilha, C.E.R., Kozak, C. et al. 20-Month monitoring of SARS-CoV-2 in wastewater of Curitiba, in Southern Brazil. Environ Sci Pollut Res 30, 76687–76701 (2023). https://doi.org/10.1007/s11356-023-27926-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27926-x