Abstract
Microglia-mediated neuroinflammation plays a vital role in the pathophysiological processes of multiple neurodegenerative diseases. Lipopolysaccharide (LPS) is an environmental poison that can induce inflammatory microglial activation. Matrix metalloproteinases (MMPs) are vital factors regulating microglial activation, and CD147 is a key MMP inducer, which can induce inflammation by inducing MMPs. However, whether it is involved in the regulation of microglial activation has not been reported. In this study, the role of CD147 in LPS-induced microglial inflammatory activation was investigated by establishing in vivo and in vitro models. The results suggested that LPS-induced microglial activation was accompanied by the induction of CD147 expression while the inhibition of CD147 expression could inhibit LPS-induced microglial inflammatory activation. In addition, the results also indicated that the role of CD147 in LPS-induced pro-inflammatory activation of microglia was related to its downstream MMP-3, MMP-8, and autophagy. Furthermore, the inhibition of MMP-3, MMP-8, and autophagy attenuated LPS-induced inflammatory activation of microglia. At the same time, there was a certain interaction between MMPs and autophagy, which is shown that inhibiting the expression of MMPs could inhibit autophagy, whereas inhibiting autophagy could inhibit the expression of MMPs. Taken together, we provided the first evidence that CD147/MMPs can be involved in LPS-induced inflammatory activation of microglia through an autophagy-dependent manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation has been recognized as a pathophysiological process associated with multiple neurological and neurodegenerative diseases, such as Alzheimer’s disease (Calsolaro and Edison 2016), Parkinson’s disease (Hirsch and Hunot 2009), and acute ischemic stroke (Lee et al. 2014b). Microglia are innate immune cells in the central nervous system (CNS), which is an important defense line of central nervous system (Hickman et al. 2018; Prinz et al. 2019). Under physiological conditions, microglia play an immune monitoring role by clearing injured neurons and pathogens, and maintaining the homeostasis of central nervous system (Gehrmann et al. 1995). Under pathological conditions, microglia switch to activated state, and the activated microglia can be classified as M1 phenotype and M2 phenotype. M1 microglia damage the central nervous system by releasing pro-inflammatory factors and neurotoxic substances. On the contrary, M2 microglia can remove dead neuron debris and degenerate synapses through immune phagocytosis, secrete anti-inflammatory cytokines, and protect the central nervous system (Perry et al. 2010). During inflammatory responses, excessive or long-term activation of microglia leads to neuronal death and increases the release of pro-inflammatory cytokines (Rodriguez-Gomez et al. 2020; Zhang et al. 2021b).
Lipopolysaccharide (LPS) is an important immunomodulatory and structural component in the outer membranes of Gram-negative bacteria (Sweeney and Lowary 2019). It is also regarded as an important environmental poison. For example, in the study of Thrasher JD et al., LPS was identified as one of 9 biological pollutants in humid indoor spaces (Thrasher and Crawley 2009). Another study by Tschernig T et al. has found that LPS from tobacco smoke and various environmental and occupational dusts can be a harmful substance that causes lung inflammation (Tschernig et al. 2008). Meanwhile, it has long been confirmed to be an environmental poison that induces neuroinflammation through the induction of microglial activation (Campbell 2004). It has been shown that LPS treatment can cause learning and memory impairment, hippocampal microglial activation, and neuron loss in mice (Ji et al. 2012; Zhao et al. 2019). In vitro studies have also confirmed that LPS activates microglia to release proinflammatory cytokines and a series of neurotoxic factors to promote neurodegenerations (Ali et al. 2022; Fan et al. 2015; Yao et al. 2019). Therefore, it is often used to establish neuroinflammatory models in vivo and in vitro.
CD147, also known as extracellular matrix metalloproteinase (MMP) inducer (EMMPRIN), HAb18G, or basigin (BSG), belongs to the immunoglobulin superfamily and is a single-chain transmembrane glycoprotein (Kosugi et al. 2015; Muramatsu and Miyauchi 2003; Yurchenko et al. 2010). CD147 has been found to be participated in multiple physiological and pathological processes, e.g., embryonic development and inflammation. CD147 is upregulated in several tumors and regulates the proliferation, apoptosis, differentiation, invasion, migration, and metastasis of tumor cells by stimulating the release of MMPs and inflammatory factors, which is related to poor prognoses (Xiong et al. 2014; Zhang et al. 2022). In addition, CD147, MMP-2, MMP-3, and MMP-9 have been found to be highly expressed in COVID-19 patients and are associated with hyperinflammation and disease severity (Springall et al. 2022). An earlier study has found that CD147-knockout mice may have spatial learning and memory impairment, suggesting that CD147 may have certain biological functions in the nervous system (Naruhashi et al. 1997). Inhibition of CD147 reduces MMP levels, attenuates histological damages, and improves long-term cognitive outcomes of elderly mice after experimental stroke (Patrizz et al. 2020). Moreover, previous studies have shown that CD147 is further involved in the regulation of macrophage-mediated inflammatory responses through regulating MMPs. For example, in atherosclerosis, the suppression of macrophage CD147 may exert an atheroprotective effect via various processes such as the clearance of LDL and plasma lipoprotein, and the decrease of platelet aggregation (Lv et al. 2020). Meanwhile, CD147 and MMP-9 are highly expressed in macrophages of atherosclerotic plaques, and the inhibition of CD147 can effectively reduce MMP-9 expression in monocyte-derived foam cells and reduces the degradation of matrix, which plays a vital role in stabilizing plaques (Seizer et al. 2010). Furthermore, a study has found that the expressions of CD147 and MMP-9 are ascended in RAW264.7 macrophages stimulated by LPS (Wang et al. 2019b). As resident immune cells of the brain, microglia are similar to macrophages in many aspects. Whether microglial pro-inflammatory activation is regulated by CD147 remains to be further clarified.
Therefore, in this study, we investigated the expression of CD147 in LPS-induced microglial inflammatory activation through in vivo and in vitro models. On this basis, the role of CD147 and its downstream effector, MMPs, in LPS-induced inflammatory activation of microglia was determined by in vitro experiments. This study aims to provide a new theoretical basis and possible targets for the intervention of neuroinflammatory injuries caused by exogenous toxicants represented by LPS.
Materials and methods
Chemicals and antibodies
LPS (from Escherichia coli O111:B4) and 3-methyladenine (3-MA) were purchased from Sigma-Aldrich Corp. (USA). MMP-3 inhibitor NNGH was purchased from APExBIO (USA), and MMP-8 inhibitor ((3R)-N-hydroxy-2-(4-methoxyphenyl)sulfonyl-3,4-dihydro-1H-isoquinoline-3-carboxamide) was bought from Santa Cruz Biotechnology (USA). Antibodies to CD147, MMP-3, MMP-8, MMP-9, MMP-14, Beclin 1, and LC3-II were supplied by Abcam (USA), ATG-5 antibody was supplied by Cell Signaling Technology (USA). IBA1 antibody was obtained from Invitrogen (USA), and Actin antibody was purchased from Sigma-Aldrich Corp. (USA).
Animal models
Our method of establishing animal models was referred to the studies of other scholars (Joshi et al. 2021; Oliveira-Lima et al. 2019; Zhang et al. 2021a). The 6-week-old male C57BL/6 mice were purchased from the Laboratory Animal Center of Army Medical University (Third Military Medical University). All mice were fed with day/night rhythm of 12/12 h (light on and off at 07:00 a.m. and 07:00 p.m.), room temperature 25 ± 1 °C, and food and water were supplied ad libitum. After 1 week of adaptive feeded, animals were randomly assigned to 2 treatment groups and intraperitoneal injection (i.p.) of normal saline (0.9% NaCl) or LPS (10 mg/kg). Mice were used for follow-up tests 24 h after LPS injection. Housing and all animal experiments were approved by the Institutional Animal Care and Use Committee of Army Medical University (Third Military Medical University).
Cell culture
BV2 microglial cell line was purchased from Shanghai Cell Bank (Chinese Academy of Sciences, China). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (HyClone, USA) with 10% fetal bovine serum (FBS) (Every Green, China) and 1% penicillin (100 U/ml)/streptomycin (100 μg/ml) (Beyotime, China) in a humidified incubator with 5% CO2 at 37 °C.
Immunofluorescence assay
After different treatments, mice were anesthetized with 10% chloral hydrate. The brains were taken out after rapid perfusion, fixed with 4% paraformaldehyde (Beyotime, China), and dehydrated in sucrose. On the cryostat, the coronal section was performed at a thickness of 20 μm. The sections were permeated with 0.3% Tritonx-100 (Beyotime) for 20 min, and then blocked with goat serum (Nanjing Jiancheng Bioengineering Institute, China) for 30 min at room temperature. The sections were incubated with mouse anti-IBA1 and rabbit anti-CD147 antibodies overnight at 4 °C. After 3 times of washing with PBS for 10 min each, the sections were incubated with fluorescent second antibodies (Alexa Fluor 488-conjugated anti-rabbit IgG or Alexa Fluor 546-conjugated anti-mouse IgG, Invitrogen, USA) for 1 h at room temperature and dark conditions. After washing again with PBS for 3 times and 10 min each time, nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI) (Beyotime, China). Sections were finally observed with a fluorescence microscope.
For cell immunofluorescence, cells were seeded on sterile glass coverslips and treated with different factors for 2 or 24 h. Then, they were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, blocked with goat serum, and incubated with CD147 and LC3-II primary antibodies. Next, the coverslips were incubated with fluorescein-labeled secondary antibodies for 1 h at room temperature, followed by nuclear counterstaining with DAPI and photographed.
Transfection and generation of stable cell lines
Mouse CD147 shRNA lentiviral interference vector and negative control (NC) were purchased from GenePharma (China). Cells were seeded in 6-well plates (1 × 105 cells/well). After attachment, cells were transfected with shRNA at a final concentration of 100 nM. The stably transfected cells were screened under 5 μg/ml polybrene. Total cell RNA was extracted for qRT-PCR to measure the expression of CD147 in the transfected cells.
RNA extraction and qRT-PCR
Total RNA was extracted with Eastep®Super Total RNA Extraction Kit (Promega, USA) according to manufacturer’s protocol. Approximately 500 ng RNA was reversely transcribed with PrimeScript TM RT Master Mix (TaKaRa, China). qRT-PCR was performed on aliquots of the cDNA preparations to detect gene expressions. RT-RCR primers from mice are presented in Table 1.
Western blot analysis
Protein extraction and western blot were carried out as previously reported (Yao et al. 2019). In this study, primary antibodies were as follows: CD147, ATG-5, Beclin 1, LC3-II, MMP-3, MMP-8, MMP-9, MMP-14, and Actin antibodies.
ELISA
Cell supernatant was collected, and the IL-6, IL-1β, and TNF-α contents were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Uscn Life Science Inc., China).
Statistical analysis
Statistical analyses for comparison of mean values were performed by One-way analysis of variance (ANOVA) followed by LSD post hoc test using SPSS 11.0 for windows. All data are presented as means ± SEM. p < 0.05 was considered statistically significant.
Results
CD147 was involved in LPS-induced proinflammatory activation of microglia in vivo and in vitro
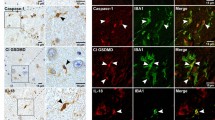
To investigate the expression of CD147 in LPS-induced mouse, immunofluorescence staining was performed. IBA1 was used as a specific marker for microglia. As shown in Fig. 1A, co-localization of CD147 with IBA1 was seen in LPS-treated mouse brains but rarely observed within control mouse brains. Furthermore, qRT-PCR and Western blot were used to detect the expression of CD147 mRNA and protein in BV2 cells after treatment with 0.5 μg/ml LPS. As shown in Fig. 1B and C, LPS significantly elevated the levels of CD147 mRNA and protein compared with those in control group. Moreover, immunofluorescence assay suggested that CD147 expression was obviously elevated in LPS-treated microglia (Fig. 1D). Thus, these data indicated that LPS induced the expression of CD147 in microglia in vivo and in vitro.
High expression of microglial CD147 stimulated by LPS in vivo and in vitro. A Mice brain Sects. (20 μm thick) were prepared 24 h after LPS and saline i.p. injection (control), and the co-localization of IBA-1 (red) and CD147 (green) was assayed by immunofluorescence. Nuclei were stained with 4, 6-diamidino-2-phenylindole (DAPI). Scale bar = 200 µm. BV2 cells were stimulated with control or LPS (0.5 μg/ml) for 6 or 24 h, then the expression of CD147 mRNA and protein were detected by qRT-PCR (B) and Western blot (C). Data are shown as mean ± SEM, *p < 0.05 vs control group. (D) Immunofluorescence analysis of CD147 expression in BV2 cells after 24 h of treatment with control or LPS (0.5 μg/ml), nuclei were stained with DAPI. Scale bar = 200 µm
Then, to further address whether CD147 was involved in LPS-stimulated microglial activation, BV2 cells with downregulated CD147 expression were constructed by shRNA-CD147 lentiviral vector (Fig. 2A, B). Subsequently, the effects of CD147 interfering on LPS-induced inflammatory cytokines (IL-6, IL-1β, and TNF-α) were detected. As illustrated in Fig. 2C and D, the expression and release of proinflammatory cytokines IL-6, IL-1β, and TNF-α induced by LPS were significantly alleviated after CD147 expression was knocked down. Collectively, these results suggested that CD147 may be involved in LPS-induced microglial pro-inflammatory activation.
Knockdown of CD147 significantly inhibited LPS-induced pro-inflammatory activation of microglia in vitro. CD147 interference lentivirus was constructed and transfected into BV2 cells, the CD147 interference efficiency was measured by qRT-PCR (A) and Western blot (B). C The effect of CD147 interference on mRNA expression of IL-6, IL-1β, and TNF-α of BV2 cells after LPS (0.5 μg/ml) treatment for 6 h, as determined by qRT-PCR analysis. D The effect of CD147 interference on the release of IL-6, IL-1β, and TNF-α of BV2 cells after LPS (0.5 μg/ml) treatment for 24 h, as determined by ELISA. NC, negative control; sh-CD147, CD147 shRNA transfection group. *p < 0.05, **p < 0.01, ***p < 0.001 vs NC group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs NC + LPS group. All data are presented as mean ± SEM of three independent experiments
The role of CD147 in LPS-induced pro-inflammatory microglial activation was MMPs-dependent
In order to verify the expression of MMPs in LPS-stimulated microglia, the expressions of MMP-3, MMP-8, MMP-9, and MMP-14 in BV2 cells were detected by qRT-PCR and Western blot. As illustrated in Fig. 3A and B and supplementary Fig. 1A,B, both mRNA and protein levels of four MMPs were remarkably upregulated in LPS-treated microglia.
The effect of LPS on the expression of MMPs in microglia. BV2 cells were treated with 0.5 μg/ml LPS for 6 h and 24 h, and the mRNA and protein expressions of MMP-3 and MMP-8 were detected by qRT-PCR (A) and Western blot (B). Then, BV2 cells were pretreated with 100 μM NNGH (MMP-3 inhibitor) or MMP-8 inhibitor for 2 h and then treated with 0.5 μg/ml LPS for 6 h or 22 h, qRT-PCR and ELISA were performed to measure the mRNA expression (C, E) and release (D, F) of IL-6, IL-1β, and TNF-α. NNGH, the inhibitor of MMP-3; MMP8 I, the inhibitor of MMP-8. *p < 0.05, **p < 0.01, ***p < 0.001 vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs LPS group. Data are expressed as means ± SEM of three independent experiments
Furthermore, to explore whether these MMPs were involved in LPS-induced microglial pro-inflammatory activation by CD147, the effect of CD147 silencing on MMPs in BV2 cells stimulated by LPS was further determined. As shown in Fig. 4A-D, the mRNA and protein expressions of MMP-3 and MMP-8 were significantly induced after LPS treatment and such effect was diminished after the knock-down of CD147 expression. However, there was no significant change in the mRNA and protein expression of microglial MMP-9 and MMP-14 induced by LPS after the knockdown of CD147 expression (supplementary Fig. 1C-F). So, the data indicate that the role of CD147 in LPS-induced microglial pro-inflammatory activation might be mediated via its effects on MMP-3 and MMP-8.
The effect of CD147 knockdown on LPS-induced MMP expression. After intervention of CD147, BV2 cells were treated with LPS for 6 h or 24 h, the mRNA expressions of MMP-3 and MMP-8 were detected by qRT-PCR (A, C), and the protein expressions of MMP-3 and MMP-8 were detected by Western blot (B, D). NC, negative control; sh-CD147, CD147 shRNA transfection group. NC, negative control; sh-CD147, CD147 shRNA transfection group. *p < 0.05, **p < 0.01, ***p < 0.001 vs NC group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs NC + LPS group. Data are expressed as mean ± SEM of three independent experiments
Next, microglia were further pretreated with 100 μM NNGH (MMP-3 inhibitor) and MMP-8 inhibitor for 2 h and then stimulated with LPS. As depicted in Fig. 3C-F, the inhibition of MMP-3 and MMP-8 activity could restrain LPS-stimulated expression and release of IL-6, IL-1β, and TNF-α. These results demonstrate that MMP-3 and MMP-8 can be involved in LPS-induced pro-inflammatory microglial activation.
Autophagy can be involved in the role of CD147 in LPS-induced microglial activation
Autophagy is widely believed to be associated with multiple neurodegenerative diseases, and microglial activation is closely related to neuroinflammation (Plaza-Zabala et al. 2017; Su et al. 2016). Previous studies have reported that LPS stimulates autophagy in microglia (Qin et al. 2018; Xie et al. 2021). To further determine the role of autophagy in LPS-induced microglial activation, BV2 cells were treated with 3-MA (autophagy inhibitor) and LPS alone or together. qRT-PCR analysis demonstrated that 3-MA inhibited the mRNA expression of IL-6, IL-1β, and TNF-α in LPS-treated microglia (Fig. 5A). Next, the potential effect of CD147 expression on LPS-induced autophagy was evaluated. As illustrated in Fig. 5B, the suppression of CD147 reduced the protein expression of key autophagy genes LC3-II, ATG-5, and Beclin1 in LPS-induced microglia. Moreover, immunofluorescence assay also showed that the knockdown of CD147 significantly reduced the expression of LC3-II (Fig. 5C). These data suggested that autophagy can be involved in the role of CD147 in LPS-induced microglial activation.
The role of autophagy in the pro-inflammatory activation of microglia regulated by CD147. BV2 cells were pretreated with 30 μM 3-MA for 2 h and then stimulated with 0.5 μg/ml LPS for 6 h, the mRNA expression of IL-6, IL-1β, and TNF-α were assayed by qRT-PCR (A). 3-MA: the inhibitor of autophagy. **p < 0.01, ***p < 0.001 vs control group; #p < 0.05, ##p < 0.01 vs LPS group. The BV2 cells with sh-CD147 intervention were treated with LPS for 24 h, the protein levels of Beclin 1, ATG-5, and LC3-II were examined by Western blot (B). Data are expressed as mean ± SEM of three independent experiments. NC, negative control; sh-CD147, CD147 shRNA transfection group. *p < 0.05, **p < 0.01 vs NC group; #p < 0.05 vs NC + LPS group. C The expression of LC3-II was detected by immunofluorescence in CD147 knock-down BV2 cells after treatment with 0.5 μg/ml LPS for 24 h, then nuclei were stained with DAPI. Scale bar = 20 µm
The interaction between MMPs and autophagy during microglial activation induced by LPS
In order to validate the role of MMPs during LPS-induced autophagy, we evaluated the influence of MMP activity inhibition on LPS-induced autophagy. The results showed that compared with LPS group, NNGH significantly attenuated the protein expression of Beclin1, ATG-5, and LC3-II in LPS-stimulated microglia (Fig. 6A). Subsequently, immunofluorescence assay showed that inhibition of MMP-3 activity reduced the expression of LC3-II in LPS-treated microglia (Fig. 6C). Identically, as illustrated in Fig. 6B and D, MMP-8 inhibitor also inhibited the expression of autophagy-related genes (LC3-II, ATG-5, and Beclin1) stimulated by LPS. Further, we studied whether inhibition of autophagy affects the expression of MMP-3 and MMP-8. As illustrated in Fig. 6E and F, 3-MA markedly attenuated LPS-induced mRNA and protein expression of MMP-3 and MMP-8 stimulated by LPS. Collectively, these data suggested that there could be interaction between autophagy and MMPs in microglia in response to LPS.
The interaction between MMPs and autophagy during LPS-induced microglial activation. BV2 cells were pretreated with 100 μM NNGH or MMP-8 inhibitor for 2 h and then treated with 0.5 μg/ml LPS for 22 h. The protein levels of Beclin 1, ATG-5 and LC3-II were assayed via Western blot (A, B). C, D The immunofluorescence analysis results of LC3-II expression. Scale bar = 20 µm. BV2 cells were pretreated with 30 μM 3-MA for 2 h, then treated with 0.5 μg/ml LPS for 6 h or 24 h, the mRNA expressions of MMP-3 and MMP-8 were measured by qRT-PCR (E), and the protein expressions of MMP-3 and MMP-8 were detected by Western blot (F). NNGH: the inhibitor of MMP-3, MMP8 I: the inhibitor of MMP-8, 3-MA: the inhibitor of autophagy. *p < 0.05, **p < 0.01, ***p < 0.001 vs control group; #p < 0.05, ##p < 0.01 vs LPS group. Data are expressed as means ± SEM of three independent experiments
Discussion
In the current study, we found that LPS could increase the expression of CD147 in microglia in vivo and in vitro. Furthermore, the inhibition of CD147 expression could alleviate LPS-induced pro-inflammatory activation of microglia in vitro. In addition, the role of CD147 in LPS-induced microglial activation was related to its downstream MMP-3, MMP-8, and autophagy, as the inhibition of MMP-3, MMP-8, and autophagy could reverse LPS-induced microglial activation. Moreover, there was potential interaction between MMPs and autophagy following LPS treatment.
As an extracellular matrix metalloproteinase inducer, CD147 plays a vital role in various pathological processes related to inflammation (e.g., atherosclerosis, tumors, etc.). Recent studies have found that CD147 is highly expressed and its DNA is demethylated in non-small cell lung cancer (NSCLC), and CD147 targeting methylation can inhibit the invasion and metastasis of NSCLC (Liao et al. 2022). Furthermore, downregulation of CD147 reduces proliferation, invasion, and drug resistance of oral cancer cells (Pan et al. 2021). CD147 is also associated with multiple CNS diseases, including Alzheimer’s disease (Wang et al. 2021), multiple sclerosis (Agrawal et al. 2013), ischemic stroke (Patrizz et al. 2020), and glioma (Wang et al. 2020a). Jin et al. found that the expression of CD147 in ischemic brain endothelial cells increased rapidly after transient middle cerebral artery occlusion, and the inhibition of CD147 supressed the infarct size and neurological deficits (Jin et al. 2017). Yin et al. found that inhibition of CD147 could inhibit proliferation, invasion, and angiogenesis of glioma cell, arrest cell cycle, and induce apoptosis in glioma (Yin et al. 2017). In addition, targeting CD147 can modulate the resistance of glioma cells to chemotherapeutics (Bu et al. 2021). In this study, we preliminarily explored the role of CD147 in microglial pro-inflammatory responses induced by exogenous neurotoxins. The results showed that LPS-treated microglial activation was accompanied by up-regulation of CD147 expression, and the inhibition of CD147 expression could restrain the activation of microglia induced by LPS, suggesting that CD147 may participate in LPS-induced pro-inflammatory activation of microglia.
Matrix metalloproteinases (MMPs) are zinc-containing peptidases that can degrade extracellular matrixes. They are not only the structural scaffolds of cells in tissues, but also the mediators of cell to cell communication (Rivera et al. 2019). MMPs are involved in tumor invasion and metastasis in various tumors, and their high expression is related to poor prognosis of tumors (Conlon and Murray 2019; Sang et al. 2021). Marcaccini et al. found that the expression level of MMP-8 in periodontal tissue of patients with periodontal disease was higher than that of healthy people (Marcaccini et al. 2010), and it has been considered to be a biomarker of periodontitis stage (Hernandez et al. 2020). Lerner et al. found that MMP-3 was an important marker for the treatment and prognosis of rheumatoid arthritis (Lerner et al. 2018), and dexamethasone treatment inhibited the proliferation, migration, and gene expression of inflammatory factors, CD147, MMP-3, and MMP-9 in collagen-induced arthritis (CIA) fibroblast-like synoviocytes (Wang et al. 2020b). Knockout of MMP-3 in the central nervous system has been found to inhibit the degeneration of dopaminergic neurons in substantia nigra in MPTP-induced PD mouse model (Kim et al. 2007), while LPS stimulation promotes microglial activation and MMP-3 expression in cerebral ischemia reperfusion (CIRP) rat model, thereby aggravating neurological damages after acute stroke (Feng et al. 2021). MMP-8 degrades tight junction protein occludin in blood–brain barrier and causes neuroinflammation in bacterial meningitis (Schubert-Unkmeir et al. 2010). Previous studies have found that the expression of MMP-3 and MMP-8 expression is upregulated in LPS-stimulated microglia (Lee et al. 2015), and the inhibition of both MMP activities by inhibitors can inhibit LPS-induced release of TNF-α in microglia (Lee et al. 2017, 2014a). Our results are consistent with previous reports, which further confirmed that MMP-3 and MMP-8 were related to pro-inflammatory activation of microglia. Moreover, a recent study has found that resveratrol inhibits the activation of pro-inflammatory microglia by down regulating CD147/MMP-9, which has a protective effect against ischemic brain injury (Zhang and Zhao 2022). In this study, CD147 knockdown obviously abated the expression of MMP-3 and MMP-8 in microglia caused by LPS rather than MMP-9 and MMP-14. These results indicated that CD147 may participate in the pro-inflammatory microglial activation by the induction of MMP-3 and MMP-8, but not MMP-9 and MMP-14.
Autophagy is an important homeostasis regulatory pathway in eukaryote organisms, through which cellular materials are transported to lysosomes for degradation (Limpert et al. 2018). Neuroinflammation and autophagy dysfunction are closely associated with neurodegeneration. The role of autophagy in neuroinflammation is complex. It can mitigate the neuroinflammatory responses (Zhou et al. 2018), but autophagy dysfunction may exacerbate neuroinflammatory responses, leading to undesirable consequences (Berglund et al. 2020). The activation of microglia is closely related to neuroinflammation, and growing evidence has found that autophagy in microglia is participated in the regulation of neuroinflammation (Berglund et al. 2020; Su et al. 2016). For example, Qin et al. demonstrated that abnormal autophagy of microglia can aggravate pro-inflammatory responses of LPS and aggravate MPTP-induced neurodegeneration by regulating inflammatory responses of NLRP3 (Qin et al. 2021). Moreover, Yang et al. found that cerebral ischemia could induce autophagy activation and inflammatory responses of microglia, while 3-MA inhibited the autophagy and inflammatory responses of microglia, and significantly reduced the infarct area, the formation of edema, and neurological defects (Yang et al. 2015). Similarly, our results found that the inhibition of autophagy with 3-MA restrained LPS-induced pro-inflammatory activation of microglial, which further confirmed that autophagy was involved in LPS-induced microglial activation.
CD147 has been shown to regulate autophagy. In nonalcoholic fatty liver disease (NAFLD) mice, CD147-knockout can induce changes in liver autophagy (Lou et al. 2020). The small molecule AC-73 is a specific inhibitor of CD147, which can inhibit the proliferation of leukemia cells and induce autophagy by blocking ERK/STAT3 signaling pathway (Spinello et al. 2019). Both autophagy and MMPs are regulated by CD147. Recent studies have shown that there can be potential interaction between autophagy and MMPs. On the one hand, autophagy can be regulated by MMPs. Pratt et al. have found that MT1-MMP promotes autophagy in glioblastoma (Pratt et al. 2016). Lin et al. have found that the inhibition of MMP-9 can prevent ssTBI-induced neurodegeneration through autophagy pathway (Lin et al. 2020). On the other hand, autophagy may regulate MMPs. Wang et al. found that MMP-13 in diabetic nucleus pulposus cells was regulated by MAPK, NF-κB signaling pathways, and autophagy (Wang et al. 2019a). However, the interaction between them in LPS-induced microglial pro-inflammatory activation remains unclear. In this study, the intervention of CD147/MMP-3/MMP-8 could inhibit autophagy, and the inhibition of autophagy could also reduce the expressions of MMP-3 and MMP-8. Our findings showed that the regulation of CD147 on microglial pro-inflammatory activation may be related to its regulation of MMPs and autophagy, and there may be some interaction between MMPs and autophagy in this process.
Conclusion
In summary, our results demonstrate that CD147-mediated expression of MMP-3 and MMP-8 can be involved in LPS-induced inflammatory activation of microglia through an autophagy-dependent manner. Moreover, there may be an interaction between autophagy and MMPs. However, there are still some deficiencies in this study. For example, the role of autophagy in CD147/MMP-mediated pro-inflammatory activation of microglia has only been preliminarily studied, and the specific mechanism needs to be further clarified. Moreover, in our study, we did not explore how LPS induced the increase of CD147 expression, which is worthy of our further study. In conclusion, these results can provide new ideas for further elucidating the molecular mechanism of LPS-induced neurotoxicity and exploring new intervention strategies.
Data availability
The data used for this study are available from the corresponding authors upon request.
References
Agrawal SM, Williamson J, Sharma R et al (2013) Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain 136:1760–1777. https://doi.org/10.1093/brain/awt093
Ali F, Hossain MS, Abdeen A et al (2022) Plasmalogens ensure the stability of non-neuronal (microglial) cells during long-term cytotoxicity. Environ Sci Pollut Res Int 29:2084–2097. https://doi.org/10.1007/s11356-021-15773-7
Berglund R, Guerreiro-Cacais AO, Adzemovic MZ et al (2020) Microglial autophagy-associated phagocytosis is essential for recovery from neuroinflammation. Sci Immunol 5: eabb5077. https://doi.org/10.1126/sciimmunol.abb5077
Bu X, Qu X, Guo K et al (2021) CD147 confers temozolomide resistance of glioma cells via the regulation of beta-TrCP/Nrf2 pathway. Int J Biol Sci 17:3013–3023. https://doi.org/10.7150/ijbs.60894
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12:719–732. https://doi.org/10.1016/j.jalz.2016.02.010
Campbell A (2004) Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci 1035:117–132. https://doi.org/10.1196/annals.1332.008
Conlon GA, Murray GI (2019) Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol 247:629–640. https://doi.org/10.1002/path.5225
Fan K, Li D, Zhang Y et al (2015) The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation. J Neuroinflammation 12:54. https://doi.org/10.1186/s12974-015-0268-x
Feng D, Chen D, Chen T et al (2021) Aspirin exerts neuroprotective effects by reversing lipopolysaccharide-induced secondary brain injury and inhibiting matrix metalloproteinase-3 gene expression. Dis Markers 2021:3682034. https://doi.org/10.1155/2021/3682034
Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 20:269–287. https://doi.org/10.1016/0165-0173(94)00015-h
Hernandez M, Baeza M, Contreras J et al (2020) MMP-8, TRAP-5, and OPG levels in gcf diagnostic potential to discriminate between healthy patients’, mild and severe periodontitis sites. Biomolecules 10:1500. https://doi.org/10.3390/biom10111500
Hickman S, Izzy S, Sen P et al (2018) Microglia in neurodegeneration. Nat Neurosci 21:1359–1369. https://doi.org/10.1038/s41593-018-0242-x
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. https://doi.org/10.1016/S1474-4422(09)70062-6
Ji A, Diao H, Wang X et al (2012) n-3 polyunsaturated fatty acids inhibit lipopolysaccharide-induced microglial activation and dopaminergic injury in rats. Neurotoxicology 33:780–788. https://doi.org/10.1016/j.neuro.2012.02.018
Jin R, Xiao AY, Chen R et al (2017) Inhibition of CD147 (cluster of differentiation 147) ameliorates acute ischemic stroke in mice by reducing thromboinflammation. Stroke 48:3356–3365. https://doi.org/10.1161/STROKEAHA.117.018839
Joshi L, Plastira I, Bernhart E et al (2021) Inhibition of autotaxin and lysophosphatidic acid receptor 5 attenuates neuroinflammation in LPS-activated BV-2 microglia and a mouse endotoxemia model. Int J Mol Sci 22:8519. https://doi.org/10.3390/ijms22168519
Kim YS, Choi DH, Block ML et al (2007) A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J 21:179–187. https://doi.org/10.1096/fj.06-5865com
Kosugi T, Maeda K, Sato W et al (2015) CD147 (EMMPRIN/Basigin) in kidney diseases: from an inflammation and immune system viewpoint. Nephrol Dial Transplant 30:1097–1103. https://doi.org/10.1093/ndt/gfu302
Lee EJ, Moon PG, Baek MC et al (2014a) comparison of the effects of matrix metalloproteinase inhibitors on TNF-alpha release from activated microglia and TNF-alpha converting enzyme activity. Biomol Ther (seoul) 22:414–419. https://doi.org/10.4062/biomolther.2014.099
Lee EJ, Ko HM, Jeong YH et al (2015) beta-Lapachone suppresses neuroinflammation by modulating the expression of cytokines and matrix metalloproteinases in activated microglia. J Neuroinflammation 12:133. https://doi.org/10.1186/s12974-015-0355-z
Lee EJ, Choi MJ, Lee G et al (2017) Regulation of neuroinflammation by matrix metalloproteinase-8 inhibitor derivatives in activated microglia and astrocytes. Oncotarget 8: 78677–78690. https://doi.org/10.18632/oncotarget.20207
Lee Y, Lee SR, Choi SS et al (2014b) Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int 2014:297241. https://doi.org/10.1155/2014/297241
Lerner A, Neidhofer S, Reuter S et al (2018) MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best Pract Res Clin Rheumatol 32:550–562. https://doi.org/10.1016/j.berh.2019.01.006
Liao CG, Liang XH, Ke Y et al (2022) Active demethylation upregulates CD147 expression promoting non-small cell lung cancer invasion and metastasis. Oncogene 41:1780–1794. https://doi.org/10.1038/s41388-022-02213-0
Limpert AS, Lambert LJ, Bakas NA et al (2018) Autophagy in cancer: regulation by small molecules. Trends Pharmacol Sci 39:1021–1032. https://doi.org/10.1016/j.tips.2018.10.004
Lin C, Wu W, Lu H et al (2020) MMP-9 Inhibitor GM6001 prevents the development of ssTBI-induced Parkinson’s disease via the autophagy pathway. Cell Mol Neurobiol 41:1651–1633. https://doi.org/10.1007/s10571-020-00933-z
Lou J, Li C, Li ZS et al (2020) Hepatic CD147 knockout modulates liver steatosis and up-regulates autophagy in high-fat-diet-induced NAFLD mice. Biochem Biophys Res Commun 524:1010–1017. https://doi.org/10.1016/j.bbrc.2020.01.164
Lv JJ, Wang H, Cui HY et al (2020) Blockade of macrophage CD147 protects against foam cell formation in atherosclerosis. Front Cell Dev Biol 8:609090. https://doi.org/10.3389/fcell.2020.609090
Marcaccini AM, Meschiari CA, Zuardi LR et al (2010) Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J Clin Periodontol 37:180–190. https://doi.org/10.1111/j.1600-051X.2009.01512.x
Muramatsu T, Miyauchi T (2003) Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol 18: 981–7. https://doi.org/10.14670/HH-18.981
Naruhashi K, Kadomatsu K, Igakura T et al (1997) Abnormalities of sensory and memory functions in mice lacking Bsg gene. Biochem Biophys Res Commun 236:733–737. https://doi.org/10.1006/bbrc.1997.6993
Oliveira-Lima OC, Carvalho-Tavares J, Rodrigues MF et al (2019) Lipid dynamics in LPS-induced neuroinflammation by DESI-MS imaging. Brain Behav Immun 79:186–194. https://doi.org/10.1016/j.bbi.2019.01.029
Pan S, Su Y, Sun B et al (2021) Knockout of CD147 inhibits the proliferation, invasion, and drug resistance of human oral cancer CAL27 cells in Vitro and in Vivo. Int J Biol Macromol 181:378–389. https://doi.org/10.1016/j.ijbiomac.2021.03.102
Patrizz A, Doran SJ, Chauhan A et al (2020) EMMPRIN/CD147 plays a detrimental role in clinical and experimental ischemic stroke. Aging (Albany NY) 12: 5121–5139. https://doi.org/10.18632/aging.102935
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6:193–201. https://doi.org/10.1038/nrneurol.2010.17
Plaza-Zabala A, Sierra-Torre V, Sierra A (2017) Autophagy and microglia: novel partners in neurodegeneration and aging. Int J Mol Sci 18:598. https://doi.org/10.3390/ijms18030598
Pratt J, Iddir M, Bourgault S et al (2016) Evidence of MTCBP-1 interaction with the cytoplasmic domain of MT1-MMP: Implications in the autophagy cell index of high-grade glioblastoma. Mol Carcinog 55:148–160. https://doi.org/10.1002/mc.22264
Prinz M, Jung S, Priller J (2019) microglia biology: one century of evolving concepts. Cell 179:292–311. https://doi.org/10.1016/j.cell.2019.08.053
Qin C, Liu Q, Hu ZW et al (2018) Microglial TLR4-dependent autophagy induces ischemic white matter damage via STAT1/6 pathway. Theranostics 8:5434–5451. https://doi.org/10.7150/thno.27882
Qin Y, Qiu J, Wang P et al (2021) Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain Behav Immun 91:324–338. https://doi.org/10.1016/j.bbi.2020.10.010
Rivera S, Garcia-Gonzalez L, Khrestchatisky M et al (2019) Metalloproteinases and their tissue inhibitors in Alzheimer’s disease and other neurodegenerative disorders. Cell Mol Life Sci 76:3167–3191. https://doi.org/10.1007/s00018-019-03178-2
Rodriguez-Gomez JA, Kavanagh E, Engskog-Vlachos P et al (2020) Microglia: agents of the CNS pro-inflammatory response. Cells 9:1717. https://doi.org/10.3390/cells9071717
Sang C, Song Y, Jin TW et al (2021) Bisphenol A induces ovarian cancer cell proliferation and metastasis through estrogen receptor-alpha pathways. Environ Sci Pollut Res Int 28:36060–36068. https://doi.org/10.1007/s11356-021-13267-0
Schubert-Unkmeir A, Konrad C, Slanina H et al (2010) Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog 6:e1000874. https://doi.org/10.1371/journal.ppat.1000874
Seizer P, Schonberger T, Schott M et al (2010) EMMPRIN and its ligand cyclophilin A regulate MT1-MMP, MMP-9 and M-CSF during foam cell formation. Atherosclerosis 209:51–57. https://doi.org/10.1016/j.atherosclerosis.2009.08.029
Spinello I, Saulle E, Quaranta MT et al (2019) The small-molecule compound AC-73 targeting CD147 inhibits leukemic cell proliferation, induces autophagy and increases the chemotherapeutic sensitivity of acute myeloid leukemia cells. Haematologica 104:973–985. https://doi.org/10.3324/haematol.2018.199661
Springall R, Gonzalez-Flores J, Garcia-Avila C et al (2022) Elevated levels of soluble CD147 are associated with hyperinflammation and disease severity in COVID-19: a proof-of-concept clinical study. Arch Immunol Ther Exp (warsz) 70:18. https://doi.org/10.1007/s00005-022-00657-6
Su P, Zhang J, Wang D et al (2016) The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience 319:155–167. https://doi.org/10.1016/j.neuroscience.2016.01.035
Sweeney RP, Lowary TL (2019) New insights into lipopolysaccharide assembly and export. Curr Opin Chem Biol 53:37–43. https://doi.org/10.1016/j.cbpa.2019.07.004
Thrasher JD, Crawley S (2009) The biocontaminants and complexity of damp indoor spaces: more than what meets the eyes. Toxicol Ind Health 25:583–615. https://doi.org/10.1177/0748233709348386
Tschernig T, Janardhan KS, Pabst R et al (2008) Lipopolysaccharide induced inflammation in the perivascular space in lungs. J Occup Med Toxicol 3:17. https://doi.org/10.1186/1745-6673-3-17
Wang H, Lv JJ, Zhao Y et al (2021) Endothelial genetic deletion of CD147 induces changes in the dual function of the blood-brain barrier and is implicated in Alzheimer’s disease. CNS Neurosci Ther 27:1048–1063. https://doi.org/10.1111/cns.13659
Wang J, Hu J, Chen X et al (2019a) BRD4 inhibition regulates MAPK, NF-kappaB signals, and autophagy to suppress MMP-13 expression in diabetic intervertebral disc degeneration. FASEB J 33:11555–11566. https://doi.org/10.1096/fj.201900703R
Wang P, Wang Z, Yan Y et al (2020a) Psychological stress up-regulates CD147 expression through beta-arrestin1/ERK to promote proliferation and invasiveness of glioma cells. Front Oncol 10:571181. https://doi.org/10.3389/fonc.2020.571181
Wang Q, Xu B, Fan K et al (2020b) Inflammation suppression by dexamethasone via inhibition of CD147-mediated NF-kappaB pathway in collagen-induced arthritis rats. Mol Cell Biochem 473:63–76. https://doi.org/10.1007/s11010-020-03808-5
Wang YQ, Zhang J, Zhu LX et al (2019b) Positive correlation between activated CypA/CD147 signaling and MMP-9 expression in mice inflammatory periapical lesion. Biomed Res Int 2019:8528719. https://doi.org/10.1155/2019/8528719
Xie H, Lu F, Liu W et al (2021) Remimazolam alleviates neuropathic pain via regulating bradykinin receptor B1 and autophagy. J Pharm Pharmacol 73:1643–1651. https://doi.org/10.1093/jpp/rgab080
Xiong L, Edwards CK 3rd, Zhou L (2014) The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int J Mol Sci 15:17411–17441. https://doi.org/10.3390/ijms151017411
Yang Z, Zhong L, Zhong S et al (2015) Hypoxia induces microglia autophagy and neural inflammation injury in focal cerebral ischemia model. Exp Mol Pathol 98:219–224. https://doi.org/10.1016/j.yexmp.2015.02.003
Yao C, Liu X, Zhou Z et al (2019) Melatonin attenuates expression of cyclooxygenase-2 (COX-2) in activated microglia induced by lipopolysaccharide (LPS). J Toxicol Environ Health A 82:437–446. https://doi.org/10.1080/15287394.2019.1615019
Yin H, Shao Y, Chen X (2017) The effects of CD147 on the cell proliferation, apoptosis, invasion, and angiogenesis in glioma. Neurol Sci 38:129–136. https://doi.org/10.1007/s10072-016-2727-2
Yurchenko V, Constant S, Eisenmesser E et al (2010) Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol 160:305–317. https://doi.org/10.1111/j.1365-2249.2010.04115.x
Zhang H, Zhao W (2022) Resveratrol alleviates ischemic brain injury by inhibiting the activation of pro-inflammatory microglia via the CD147/MMP-9 pathway. J Stroke Cerebrovasc Dis 31:106307. https://doi.org/10.1016/j.jstrokecerebrovasdis.2022.106307
Zhang J, Wang Z, Zhang X et al (2022) Large-scale single-cell and bulk sequencing analyses reveal the prognostic value and immune aspects of CD147 in pan-cancer. Front Immunol 13:810471. https://doi.org/10.3389/fimmu.2022.810471
Zhang L, Jiang X, Zhang J et al (2021a) (-)-Syringaresinol suppressed LPS-induced microglia activation via downregulation of NF-kappaB p65 signaling and interaction with ERbeta. Int Immunopharmacol 99:107986. https://doi.org/10.1016/j.intimp.2021.107986
Zhang W, Fan X, Fan Z et al (2021b) Acute exposure to paraquat affects the phenotypic differentiation of substantia nigra microglia in rats. Environ Sci Pollut Res Int 29:21339–21347. https://doi.org/10.1007/s11356-021-17262-3
Zhao J, Bi W, Xiao S et al (2019) Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep 9:5790. https://doi.org/10.1038/s41598-019-42286-8
Zhou Q, Fu X, Wang X et al (2018) Autophagy plays a protective role in Mn-induced toxicity in PC12 cells. Toxicology 394:45–53. https://doi.org/10.1016/j.tox.2017.12.001
Funding
This study was supported by National Natural Science Foundation of China under Grant 81673211 and 81372952, Natural Science Foundation of Chongqing under cstc2018jcyjAX0311 and cstc2018jcyjAX0724, and Medical Science and Technology Youth Cultivation Project of PLA under 20QNPY017.
Author information
Authors and Affiliations
Contributions
Chunyan Yao and Xiaoling Liu: Participated in the main experiment of this study, analyzed the data, and co-wrote the manuscript.
Yan Tang and Chunmei Wang: conceived the study, methodology.
Chenggang Duan and Xiaoyan Liu: analyzed the data.
Mingliang Chen, Yumeng Zhou and Enjie Tang: cell culture, validation.
Ying Xiang and Yafei Li: supervised and managed the study.
Ailing Ji and Tongjian Cai: designed and supervised the study, acquired the funding, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Army Medical University (Third Military Medical University).
Consent to participate
All authors have read and approved the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11356_2022_24292_MOESM1_ESM.pdf
Supplementary file1 (PDF 96 kb) Supplementary Fig.1. The effect of LPS on MMP-9 and MMP-14 expression and the potential effect of CD147 knockdown in BV2 cells. After treatment with 0.5 μg/ml LPS for 6 h and 24 h, qRT-PCR and Western blot were performed to determine the mRNA (A) and protein (B) expression of MMP-9 and MMP-14. Furthermore, after the intervention of CD147, qRT-PCR (C,E) was performed to detect the mRNA expressions of MMP-9 and MMP-14 after LPS treatment for 6 h, and Western blot (D,F) was performed to detect the protein expressions of MMP-9 and MMP-14 after LPS treatment for 24 h. NC: negative control, sh-CD147: CD147 shRNA transfection group. *p < 0.05, **p < 0.01 vs NC group. Data are expressed as mean ± SEM of three independent experiments.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, C., Liu, X., Tang, Y. et al. Lipopolysaccharide induces inflammatory microglial activation through CD147-mediated matrix metalloproteinase expression. Environ Sci Pollut Res 30, 35352–35365 (2023). https://doi.org/10.1007/s11356-022-24292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24292-y