Abstract
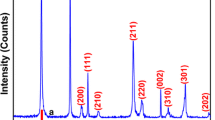
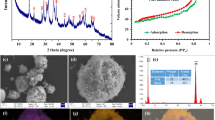
MnO2, as a representative manganese-based catalyst with many kinds of crystal forms, has been widely used to activate PMS. However, the role of morphological scale and crystal structures on the catalytic capability of MnO2 still lacks further study. In this study, four different crystal forms of MnO2 (α-MnO2, β-MnO2, γ-MnO2, and δ-MnO2) are succeeded in being fabricated via hydrothermal processes and evaluated by activating PMS for the removal of Reactive Yellow X-RG, typical azo dye. Experiment results indicate that α-MnO2 with a one-dimensional structure exhibits the best catalytic performance among the four as-prepared MnO2, which can be attributed to its broadest crystal interplanar distance (0.692), the highest portion of Mn (III)/Mn (IV) (4.194), and lowest value of average oxidation state AOS (2.696). Correlation analysis confirms that interplanar distance is the most relative factor with the catalytic activity of MnO2 among the three studied factors (R2 = 0.99715). Meanwhile, the morphological scale structure of α-MnO2 can also account for its highest catalytic ability among the four as-prepared MnO2, including its large specific area and advantageous one-dimensional nanostructure. Furthermore, according to the response surface methodology, when the dosage of PMS is 2.369 g/L, the dosage of α-MnO2 is 0.991 g/L, and the initial dye concentration is 1025 mg/L, the maximum removal rate of Reactive Yellow X-RG is up to 97.38%.
Graphical abstract









Similar content being viewed by others
Data availability
Not available.
References
Chen B, Wu B, Yu L, Crocker M, Shi C (2020) Investigation into the catalytic roles of various oxygen species over different crystal phases of MnO2 for C6H6 and HCHO oxidation. ACS Catal 10:6176–6187. https://doi.org/10.1021/acscatal.0c00459
Chen G, Zhang X, Gao Y, Zhu G, Cheng Q, Cheng X (2019) Novel magnetic MnO2/MnFe2O4 nanocomposite as a heterogeneous catalyst for activation of peroxymonosulfate (PMS) toward oxidation of organic pollutants. Sep Purif Technol 213:456–464. https://doi.org/10.1016/j.seppur.2018.12.049
Chen X, Chen J, Qiao X, Wang D, Cai X (2008) Performance of nano-Co3O4/peroxymonosulfate system: kinetics and mechanism study using Acid Orange 7 as a model compound. Appl Catal B 80:116–121. https://doi.org/10.1016/j.apcatb.2007.11.009
Cockayne E, Li L (2012) First-principles DFT+U studies of the atomic, electronic, and magnetic structure of α-MnO2 (cryptomelane). Chem Phys Lett 544:53–58. https://doi.org/10.1016/j.cplett.2012.06.061
Dai Y, Huang J, Zhang H, Liu CC (2019) Highly sensitive electrochemical analysis of tunnel structured MnO2 nanoparticle-based sensors on the oxidation of nitrite. Sens Actuators, B Chem 281:746–750. https://doi.org/10.1016/j.snb.2018.11.014
Deng Y, Zhao R (2015) Advanced oxidation processes (AOPs) in wastewater treatment. Current Pollution Reports 1:167–176. https://doi.org/10.1007/s40726-015-0015-z
Ding Y, Wang X, Fu L, Peng X, Pan C, Mao Q, Wang C, Yan J (2021) Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): recent advances and perspective. Sci Total Environ 765:142794. https://doi.org/10.1016/j.scitotenv.2020.142794
Du J, Bao J, Liu Y, Ling H, Zheng H, Kim SH, Dionysiou DD (2016) Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A. J Hazard Mater 320:150–159. https://doi.org/10.1016/j.jhazmat.2016.08.021
Eslami A, Hashemi M, Ghanbari F (2018) Degradation of 4-chlorophenol using catalyzed peroxymonosulfate with nano-MnO2/UV irradiation: toxicity assessment and evaluation for industrial wastewater treatment. J Clean Prod 195:1389–1397. https://doi.org/10.1016/j.jclepro.2018.05.137
Gao W, Ran C, Wang M, Li L, Sun Z, Yao X (2016) The role of reduction extent of graphene oxide in the photocatalytic performance of Ag/AgX (X = Cl, Br)/rGO composites and the pseudo-second-order kinetics reaction nature of the Ag/AgBr system. Phys Chem Chem Phys 18:18219–18226. https://doi.org/10.1039/c6cp03110b
Gao W, Wang M, Ran C, Yao X, Yang H, Liu J, He D, Bai J (2014) One-pot synthesis of Ag/r-GO/TiO2 nanocomposites with high solar absorption and enhanced anti-recombination in photocatalytic applications. Nanoscale 6:5498–5508. https://doi.org/10.1039/c3nr05466g
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62. https://doi.org/10.1016/j.cej.2016.10.064
Guo Y, Kong L, Lei M, Xin Y, Zuo Y, and Chen W (2021) Effect of crystallographic structure of MnO2 on degradation of 2-CEES. Journal of Molecular Liquids 333https://doi.org/10.1016/j.molliq.2021.115946
Hayashi E, Yamaguchi Y, Kamata K, Tsunoda N, Kumagai Y, Oba F, Hara M (2019) Effect of MnO2 crystal structure on aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. J Am Chem Soc 141:890–900. https://doi.org/10.1021/jacs.8b09917
Hu Y, Zhang T, Jiang L, Yao S, Ye H, Lin K, Cui C (2019a) Removal of sulfonamide antibiotic resistant bacterial and intracellular antibiotic resistance genes by UVC-activated peroxymonosulfate. Chem Eng J 368:888–895. https://doi.org/10.1016/j.cej.2019.02.207
Hu Z, Mi R, Yong X, Liu S, Li D, Li Y, Zhang T (2019b) Effect of crystal phase of MnO2 with similar nanorod-shaped morphology on the catalytic performance of benzene combustion. ChemistrySelect 4:473–480. https://doi.org/10.1002/slct.201802033
Huang C, Wang Y, Gong M, Wang W, Mu Y, and Hu Z-H (2020) α-MnO2/palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of rhodamine B. Separation and Purification Technology 230. https://doi.org/10.1016/j.seppur.2019.115877
Huang J, Dai Y, Singewald K, Liu C-C, Saxena S, Zhang H (2019) Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem Eng J 370:906–915. https://doi.org/10.1016/j.cej.2019.03.238
Huang J, and Zhang H (2019) Oxidant or catalyst for oxidation? The role of manganese oxides in the activation of peroxymonosulfate (PMS). Frontiers of Environmental Science & Engineering 13. https://doi.org/10.1007/s11783-019-1158-8
Huang J, Zhong S, Dai Y, Liu CC, Zhang H (2018) Effect of MnO2 phase structure on the oxidative reactivity toward bisphenol A degradation. Environ Sci Technol 52:11309–11318. https://doi.org/10.1021/acs.est.8b03383
Huang Y, Wang Z, Fang C, Liu W, Lou X, Liu J (2016) Importance of reagent addition order in contaminant degradation in an Fe(ii)/PMS system. RSC Adv 6:70271–70276. https://doi.org/10.1039/c6ra14081e
Ji H, Gong Y, Duan J, Zhao D, Liu W (2018) Degradation of petroleum hydrocarbons in seawater by simulated surface-level atmospheric ozone: reaction kinetics and effect of oil dispersant. Mar Pollut Bull 135:427–440. https://doi.org/10.1016/j.marpolbul.2018.07.047
Ji H, Zhu Y, Duan J, Liu W, Zhao D (2019) Reductive immobilization and long-term remobilization of radioactive pertechnetate using bio-macromolecules stabilized zero valent iron nanoparticles. Chin Chem Lett 30:2163–2168. https://doi.org/10.1016/j.cclet.2019.06.004
Jiang L, Gu Y, Guo H, Liu L, Chen J (2017) Efficient removal of 17α-ethinylestradiol (EE2) from water using freshly formed Fe–Mn binary oxide. RSC Adv 7:23802–23811. https://doi.org/10.1039/c7ra02022h
Jiang XH, Zhang LS, Liu HY, Wu DS, Wu FY, Tian L, Liu LL, Zou JP, Luo SL, Chen BB (2020) Silver single atom in carbon nitride catalyst for highly efficient photocatalytic hydrogen evolution. Angew Chem Int Ed Engl 59:23112–23116. https://doi.org/10.1002/anie.202011495
Khan A, Wang H, Liu Y, Jawad A, Ifthikar J, Liao Z, Wang T, Chen Z (2018a) Highly efficient α-Mn2O3@α-MnO2-500 nanocomposite for peroxymonosulfate activation: comprehensive investigation of manganese oxides. J Mater Chem A 6:1590–1600. https://doi.org/10.1039/c7ta07942g
Khan A, Zou S, Wang T, Ifthikar J, Jawad A, Liao Z, Shahzad A, Ngambia A, Chen Z (2018b) Facile synthesis of yolk shell Mn2O3@Mn5O8 as an effective catalyst for peroxymonosulfate activation. Phys Chem Chem Phys 20:13909–13919. https://doi.org/10.1039/c8cp02080a
Kim E-J, Oh D, Lee C-S, Gong J, Kim J, Chang Y-S (2017) Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH: crystal phase-dependent behavior. Catal Today 282:71–76. https://doi.org/10.1016/j.cattod.2016.03.034
Kong F, Zhang H, Chai H, Liu B, Cao Y (2021) Insight into the crystal structures and surface property of manganese oxide on CO catalytic oxidation performance. Inorg Chem 60:5812–5820. https://doi.org/10.1021/acs.inorgchem.1c00144
Korotcenkov G (2008) The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater Sci Eng R Rep 61:1–39. https://doi.org/10.1016/j.mser.2008.02.001
Li K, Chen C, Zhang H, Hu X, Sun T, and Jia J (2019) Effects of phase structure of MnO2 and morphology of δ-MnO2 on toluene catalytic oxidation. Applied Surface Science 496. https://doi.org/10.1016/j.apsusc.2019.143662
Li Q, Huang X, Su G, Zheng M, Huang C, Wang M, Ma C, Wei D (2018) The regular/persistent free radicals and associated reaction mechanism for the degradation of 1,2,4-trichlorobenzene over different MnO2 polymorphs. Environ Sci Technol 52:13351–13360. https://doi.org/10.1021/acs.est.8b03789
Liu Y, Qu R, Li X, Wei Y, Feng L (2020) A bifunctional beta-MnO2 mesh for expeditious and ambient degradation of dyes in activation of peroxymonosulfate (PMS) and simultaneous oil removal from water. J Colloid Interface Sci 579:412–424. https://doi.org/10.1016/j.jcis.2020.06.073
Lyu C, He D, Mou Z, Yang X (2019) Synergetic activation of peroxymonosulfate by MnO2-loaded beta-FeOOH catalyst for enhanced degradation of organic pollutant in water. Sci Total Environ 693:133589. https://doi.org/10.1016/j.scitotenv.2019.133589
Meng Y, Song W, Huang H, Ren Z, Chen SY, Suib SL (2014) Structure-property relationship of bifunctional MnO2 nanostructures: highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J Am Chem Soc 136:11452–11464. https://doi.org/10.1021/ja505186m
Mondal SK, Saha AK, Sinha A (2018) Removal of ciprofloxacin using modified advanced oxidation processes: kinetics, pathways and process optimization. J Clean Prod 171:1203–1214. https://doi.org/10.1016/j.jclepro.2017.10.091
Nawaz F, Cao H, Xie Y, Xiao J, Chen Y, Ghazi ZA (2017) Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol. Chemosphere 168:1457–1466. https://doi.org/10.1016/j.chemosphere.2016.11.138
Peng W-c, Wang S-b, Li X-y (2016) Shape-controlled synthesis of one-dimensional α-MnO2 nanocrystals for organic detection and pollutant degradation. Sep Purif Technol 163:15–22. https://doi.org/10.1016/j.seppur.2016.01.050
Sabri M, King HJ, Gummow RJ, Lu X, Zhao C, Oelgemöller M, Chang SLY, Hocking RK (2018) Oxidant or catalyst for oxidation? A study of how structure and disorder change the selectivity for direct versus catalytic oxidation mediated by manganese(III, IV) oxides. Chem Mater 30:8244–8256. https://doi.org/10.1021/acs.chemmater.8b03661
Santos VP, Pereira MFR, Órfão JJM, Figueiredo JL (2010) The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl Catal B 99:353–363. https://doi.org/10.1016/j.apcatb.2010.07.007
Saputra E, Muhammad S, Sun H, Ang HM, Tade MO, Wang S (2013) Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environ Sci Technol 47:5882–5887. https://doi.org/10.1021/es400878c
Saputra E, Muhammad S, Sun H, Patel A, Shukla P, Zhu ZH, Wang S (2012) α-MnO2 activation of peroxymonosulfate for catalytic phenol degradation in aqueous solutions. Catal Commun 26:144–148. https://doi.org/10.1016/j.catcom.2012.05.014
Sun M, Lan B, Lin T, Cheng G, Ye F, Yu L, Cheng X, and Zheng X (2013) Controlled synthesis of nanostructured manganese oxide: crystalline evolution and catalytic activities. CrystEngComm 15https://doi.org/10.1039/c3ce40603b
Wan J, Zhou L, Deng H, Zhan F, Zhang R (2015) Oxidative degradation of sulfamethoxazole by different MnO2 nanocrystals in aqueous solution. J Mol Catal a: Chem 407:67–74. https://doi.org/10.1016/j.molcata.2015.06.026
Wang J, Wang S (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J 334:1502–1517. https://doi.org/10.1016/j.cej.2017.11.059
Wang T, Zhou Y, Cao S, Lu J, Zhou Y (2019) Degradation of sulfanilamide by Fenton-like reaction and optimization using response surface methodology. Ecotoxicol Environ Saf 172:334–340. https://doi.org/10.1016/j.ecoenv.2019.01.106
Wang Y, Wu Y, Yu Y, Pan T, Li D, Lambropoulou D, Yang X (2020a) Natural polyphenols enhanced the Cu(II)/peroxymonosulfate (PMS) oxidation: the contribution of Cu(III) and HO(*). Water Res 186:116326. https://doi.org/10.1016/j.watres.2020.116326
Wang Z, Jia H, Zheng T, Dai Y, Zhang C, Guo X, Wang T, and Zhu L (2020b) Promoted catalytic transformation of polycyclic aromatic hydrocarbons by MnO2 polymorphs: synergistic effects of Mn3+ and oxygen vacancies. Applied Catalysis B: Environmental 272. https://doi.org/10.1016/j.apcatb.2020.119030
Wang Z, Wang Z, Li W, Lan Y, and Chen C (2022) Performance comparison and mechanism investigation of Co3O4-modified different crystallographic MnO2 (α, β, γ, and δ) as an activator of peroxymonosulfate (PMS) for sulfisoxazole degradation. Chemical Engineering Journal 427. https://doi.org/10.1016/j.cej.2021.130888
Xie Y, Yu Y, Gong X, Guo Y, Guo Y, Wang Y, Lu G (2015) Effect of the crystal plane figure on the catalytic performance of MnO2 for the total oxidation of propane. CrystEngComm 17:3005–3014. https://doi.org/10.1039/c5ce00058k
Yang J, Wang J, Ma S, Ke B, Yu L, Zeng W, Li Y, Wang J (2019) Insight into the effect of crystalline structure on the oxygen reduction reaction activities of one-dimensional MnO2. Physica E 109:191–197. https://doi.org/10.1016/j.physe.2018.07.032
Yang S, Wang P, Yang X, Shan L, Zhang W, Shao X, Niu R (2010) Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: persulfate, peroxymonosulfate and hydrogen peroxide. J Hazard Mater 179:552–558. https://doi.org/10.1016/j.jhazmat.2010.03.039
Yin R, Guo W, Wang H, Du J, Zhou X, Wu Q, Zheng H, Chang J, Ren N (2018) Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: performances and mechanisms. Chem Eng J 335:145–153. https://doi.org/10.1016/j.cej.2017.10.063
Yu F, Wang L, Xing Q, Wang D, Jiang X, Li G, Zheng A, Ai F, Zou J-P (2020a) Functional groups to modify g-C3N4 for improved photocatalytic activity of hydrogen evolution from water splitting. Chin Chem Lett 31:1648–1653. https://doi.org/10.1016/j.cclet.2019.08.020
Yu J, Zeng T, Wang H, Zhang H, Sun Y, Chen L, Song S, Li L, and Shi H (2020b) Oxygen-defective MnO2−x rattle-type microspheres mediated singlet oxygen oxidation of organics by peroxymonosulfate activation. Chemical Engineering Journal 394. https://doi.org/10.1016/j.cej.2020.124458
Yu L, Wang C, Ren X, Sun H (2014) Catalytic oxidative degradation of bisphenol A using an ultrasonic-assisted tourmaline-based system: influence factors and mechanism study. Chem Eng J 252:346–354. https://doi.org/10.1016/j.cej.2014.05.014
Zhang J, Li Y, Wang L, Zhang C, He H (2015) Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal Sci Technol 5:2305–2313. https://doi.org/10.1039/c4cy01461h
Zhang LS, Jiang XH, Zhong ZA, Tian L, Sun Q, Cui YT, Lu X, Zou JP, Luo SL (2021) Carbon nitride supported high-loading Fe single-atom catalyst for activation of peroxymonosulfate to generate (1) O2 with 100% selectivity. Angew Chem Int Ed Engl 60:21751–21755. https://doi.org/10.1002/anie.202109488
Zhang W, Yang Z, Wang X, Zhang Y, Wen X, Yang S (2006) Large-scale synthesis of β-MnO2 nanorods and their rapid and efficient catalytic oxidation of methylene blue dye. Catal Commun 7:408–412. https://doi.org/10.1016/j.catcom.2005.12.008
Zhou Z-G, Du H-M, Dai Z, Mu Y, Tong L-L, Xing Q-J, Liu S-S, Ao Z, Zou J-P (2019) Degradation of organic pollutants by peroxymonosulfate activated by MnO2 with different crystalline structures: catalytic performances and mechanisms. Chem Eng J 374:170–180. https://doi.org/10.1016/j.cej.2019.05.170
Zhu M, Zhang L, Liu S, Wang D, Qin Y, Chen Y, Dai W, Wang Y, Xing Q, Zou J (2020) Degradation of 4-nitrophenol by electrocatalysis and advanced oxidation processes using Co3O4@C anode coupled with simultaneous CO2 reduction via SnO2/CC cathode. Chin Chem Lett 31:1961–1965. https://doi.org/10.1016/j.cclet.2020.01.017
Zou J-P, Wu D-D, Luo J, Xing Q-J, Luo X-B, Dong W-H, Luo S-L, Du H-M, Suib SL (2016) A strategy for one-pot conversion of organic pollutants into useful hydrocarbons through coupling photodegradation of MB with photoreduction of CO2. ACS Catal 6:6861–6867. https://doi.org/10.1021/acscatal.6b01729
Funding
The authors gratefully acknowledge the support from the National Key Technology R&D Program (2017YFD0300503), the Applied Basic Research Programs of Science and Technology Commission Foundation of Heilongjiang Province (No. GC13B111), and the Natural Science Foundation of China (Nos. 31470550, 515080).
Author information
Authors and Affiliations
Contributions
Junwei Wang: writing—original draft, methodology, validation, visualization, formal analysis. Di Zhang: writing—supervision, methodology, visualization, funding acquisition. Fan Nie: writing—review and editing, visualization. Ruixue Zhang: writing—review and editing, formal analysis. Xiaojie Fang: writing—review and editing. Yaxin Wang: review and editing.
Corresponding author
Ethics declarations
Ethics approval
No human or animal data were used for the research work.
Consent to participate
Not applicable.
Consent for publication.
All authors approve the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The effects of manganese dioxide’s morphological structure and crystal structure on the activation of peroxymonosulfate (PMS) for Reactive Yellow X-RG degradation were investigated in detail.
• The influence degree of the factors that correlated with the reactivity of MnO2 descended in the sequence of crystal interplanar distance (R2 = 0.99715) > ratio of Mn (III)/Mn (IV) (R2 = 0.96783) > value of average oxidation state (AOS) (R2 = 0.62393).
• With the α-MnO2 dosage of 0.991 g/L, PMS dosage of 2.369 g/L, and dye concentration of 1025 mg/L at 20 °C, the maximum degradation rate of Reactive Yellow X-RG was obtained.
• α-MnO2 still exhibited great catalytic ability and stability after eight cycle experiments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Zhang, D., Nie, F. et al. The role of MnO2 crystal morphological scale and crystal structure in selective catalytic degradation of azo dye. Environ Sci Pollut Res 30, 15377–15391 (2023). https://doi.org/10.1007/s11356-022-23223-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23223-1