Abstract
The present study evaluated the potential for biogas generation from microalgae (MA) biomass and macrophytes used in vertical flow constructed wetlands (VFCW). The samples were obtained by separation and collection of MA after a hydraulic retention time of 14 days, frozen and taken to the laboratory, while the macrophytes of VFCW were obtained, by pruning, every 6 months. The obtained results presented reductions of 63.22% and 61.18% for COD and BOD5, respectively, and removal efficiencies of 53.91% for TP and 99.98% de N-NH3. Average biogas generation was 2322.51 NmL-gSV−1 with 54.61% CH4 (winter/2019), 4491.47 Nml-gSV−1 with 57.17% CH4 (spring/2019), 680.78 NmL-gSV−1 with 16.04% CH4 (summer/2020), and 681.0 NmL-gSV−1 with 19.86% CH4 (autumn/2020) for MA biomass and generation of biogas of 3826.70 NmL-gSV−1 with 44.26% CH4 for VFCW biomass in winter and spring/2019 and of 829.68 NmL-gSV−1 with 17.06% CH4 in summer and autumn/2020. Regarding electricity generation, the present work obtained 1.50 kWh/m3, therefore reaching similar values to other studies that used more traditional biomass sources.
Similar content being viewed by others
Introduction
The growing search for energy generation alternatives to meet the global demand has increasingly raised the number of researches and debates in the scientific and technological spheres. Although fossil fuels are responsible for meeting most of this demand, factors such as environmental degradation and limited reserves make the use of this energy matrix finite and potentially polluting (Pacheco et al. 2015; Hasan et al. 2018 and Martens et al. 2021).
Dependence on energy will always be an extremely important point for society regarding the development and maintenance of its basic activities. Over the last years, the demand for energy has grown, thus increasing the need to diversify the sources of energy generation and supply. In this context, renewable energies can mitigate greenhouse gas (GHGs) emissions as well as more sustainable energy generation (Callegari et al. 2020). Therefore, the generation of biogas through urban and agro-industrial organic waste emerges as a renewable energy alternative in the context bioenergy generation (Dalpaz et al. 2020).
The proposition of alternatives that may bring effective solutions to this scenario necessarily involves a transition from the current energy model to an expanded matrix of renewable energy sources. As some of the current alternatives for the most used energy sources, such as wind and solar, depend on climatic conditions, the generation of biogas through anaerobic digestion of organic matter is not directly associated with these climatological variables (Abad et al. 2015; Ardolino and Arena 2019).
Another considerable benefit of using biogas is the fact that, after biomethanation, it can be properly stored, i.e., without the presence of impurities that may lead to corrosion in long-term storage. Despite the costs associated with purification, this storing might be considered a significant advantage that can accommodate variable energy demands, making it an essential component among the available renewable energy options for the future of global energy supply. Furthermore, it can promote a significant contribution for decreasing air pollutants and GHG emissions (Hahn et al. 2014; Capodaglio et al. 2016a; Wall et al. 2016 and Ardolino et al. 2021). The generation of biogas is an important part of the carbon cycle and can be used as a potential fuel for energy production. After being purified, biogas can reach the same standards as fossil natural gas, replacing it, and providing so an alternative of renewable energy. As a result of the anaerobic digestion, the carbon content in the biomass is reduced and the nitrogen content is concentrated. Thus, the waste generated by the digestate differential can be used, for example, in soils as a biofertilizer. (Raslavičius et al. 2011; Da Ros et al. 2014; Karlsson 2014).
From the anaerobic digestion, the organic material contained in the biomass is converted into biogas, where its main constituents are methane gas (CH4) and carbon dioxide (CO2) (Konrad et al. 2016a; Ribeiro et al. 2016). Other gasses also present, although in smaller proportions, are: water vapors (H2O); hydrogen sulfide (H2S); Hydrocarbons; ammonia (NH3); oxygen (O2); carbon monoxide (CO) and nitrogen (N2) (Ryckebosch et al. 2011; Coimbra-Araújo et al. 2014).
Studies have been developed aiming to enhance the production of biogas through the combination of organic substrates in a process called co-digestion (Karlsson 2014). Co-digestion can promote a better carbon/nitrogen (C/N) ratio, the main nutrients used by microorganisms in anaerobic digestion, ensuring that all the carbon available in the medium may be consumed by microorganisms (Konrad et al. 2016a). Researches on the generation of biogas have received growing attention in recent years, since besides being used as a renewable energy source for heat and energy generation, it can also generate fuel (biomethane), thus constituting a sustainable alternative to fossil fuels (Lantz 2012; León and Martín 2016; Hasan et al. 2018). The production of biogas and biodiesel is growing rapidly, but neither has outpaced the production of bioethanol, mainly in Brazil.
In Brazil, the structural development of bioenergy emerges as a strategic opportunity, mainly for rural communities that can have a self-supply of energy in this technology. Additionally, the surplus production may be commercialized in the national energy supply system, since currently, the electricity sector allows injecting excess energy production into the distribution network (Da Silva et al. 2016; Konrad et al. 2016b). The application and development in the energy generation from biogas has been evident in Brazil. In 2020, there was a 23% increase in the volume of biogas produced and a 22% increase in the number of plants in operation, compared to 2019, demonstrating that a total of 1.8 billion m3 of biogas has been produced, per year, in these units (CIBIOGÁS 2021). Even so, the country has the potential to produce 10.9 billion m3 of biogas for short-term use (INSTITUTO 172021). Studies show that the digestion of carbon-rich animal and/or vegetable residues, with residues that complement these substrates, has been widely used by industry with positive results in the generation of biogas, considered a sustainable technology as the process does not entail a net consumption of energy; the process is usually referred to as “co-digestion” (Zhang et al. 2013; Capodaglio et al. 2016b; Hasan et al. 2018). Other studies analyzed the proposal to generate biogas as an alternative for energy recovery in consolidated phytoremediation systems, such as the wastewater treatment through the use of macrophytes used in constructed wetlands, recovering energy by plant biomass (Prochnow et al. 2005; Wöhler-Geske et al. 2016; Roj-Rojewski et al. 2019). Moreover, some researchers used microalgae in the treatment of wastewater. These can be exploited in anaerobic digestion for biogas production or in different biorefinery processes for the development of bioproducts, offering possibilities of reuse and energy recovery (Wu and Chang 2019; González-Fernández et al. 2012; Passos et al. 2014; Gutiérrez et al. 2015).
So, the search for environmentally viable solutions for the wastewater energy recovery process lead many researchers towards combining treatment systems, with the idea of minimizing costs and enhancing performance, in addition to recovering energy during the process (Capodaglio et al. 2021). Since the energy issue also corresponds to an urgent demand and requires the attention of researchers, the proposition of integrated systems with energy recovery becomes a trend in the near future. In this context, these technologies can contribute to bring effluent treatment closer to the concept of circular economy, although several studies have evaluated energy generation through the conversion of biomass generated from urban effluents, this is the first study using the biomass generated from a system that combines microalgae and constructed wetlands.
Therefore, this study evaluated the potential for biogas generation from microalgae (MA) biomass and macrophytes used in constructed wetland (CW) arising from the urban wastewater treatment process, as well as from the biogas generated in the production of electric energy as an alternative for environmental sanitation and energy recovery.
Materials and methods
Characterization of the study area and system setup
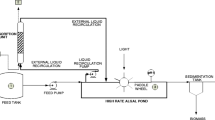
Biomass generation took place during the treatment of the urban wastewaters generated at a university campus. The integrated system was composed of an anaerobic reactor, microalgae (MA) and vertical flow constructed wetland (VFCW). The pilot project was conceived and operated at the wastewater treatment plant (WWTP) of the University of Santa Cruz do Sul/RS, (UNISC) Brazil (Fig. 1).
The WWTP was configured to meet the demands of wastewater produced by a population equivalent to 18,000 people, operating with an average flow of approximately 103 m3 day−1. Fluctuations range from 129.6 to 57.6 m3 day−1 and flow peaks occur between 12 and 17 h (Silveira et al. 2017). For this experiment, six stages of analyses were carried out at the Research Center in Energy and Sustainable Technologies (RCEST) of the University of Vale do Taquari—UNIVATES, located at the campus of Lajeado/RS, Brazil.
The first treatment stage, an anaerobic reactor (900 L), was used for partial organic matter degradation and solids settling. The microalgae tank was composed of a 200 L plastic box, in which a submerged pump was used for recirculation and a pyramid-shaped cone was inserted to support algae growth. Next, a biomass separating filter was applied aiming for the harvesting of the microalgae biomass from the wastewater. This biomass separation filter is under patent application with the National Institute of Industrial Property (registration number BR 20 2021 011,625 6). The last treatment unit was a vertical flow constructed wetland, filled with pebbles (n. 4) and gravel (n. 1) and vegetated with Hymenachne grumosa. The HRT for the treatment was 3, 7, and 7 days respectively for the AR, MA, and VFCW units, respectively. More information regarding the integrated treatment system can be found in Silveira et al. (2020).
Sampling
The project was developed and monitored from August/2018 to May/2020. As of August 2018, the prototype of the integrated system was configured and, after a period of 15 days of acclimatization, it began to be regularly monitored by physicochemical analyses. After a monitoring period of 6 months, including chemical, physical, and biological parameters, the stages of collection and analyses of the respective biomass generated during the treatment process of the evaluated effluent were programmed.
During this period, physicochemical and biological analyses were performed. Samples were obtained weekly, with analyses performed following international protocols as described in Standard Methods for the Examination of Water and Wastewater (APHA/AWWA/WPCF 2012) and Association of Official Analytical Chemists (AOAC 2000). The analyses were carried out according to a pre-determined schedule, which separated the analyses according to their frequency, being distributed as follows: monitoring analyses (bench tests), performance analysis, disinfection analyses, and toxicological analyses.
The analyses of the generated biomass were divided into seasonal periods, thus establishing four stages of collection and analyses related to microalgal biomass: winter (2019), spring (2019); summer (2019/2020), and autumn (2020). Regarding the biomass from plants used in the VFCW, collections and analyses were carried out in two stages, corresponding to the seasonal periods referring to winter and spring 2019 and summer and fall 2020.
In the first stage, microalgae biomass samples, collected with hydraulic retention time (HRT) of 14 days and during the months of June, July, and August 2019 (winter), were evaluated. The procedure was repeated for each corresponding period, September, October, and November 2019 (spring), December 2019, January and February 2020 (summer), and, finally, the period corresponding to autumn 2020, March, April, and May (Fig. 2A). The samples were preserved at frozen, being taken to the Laboratory of Bioreactors at the University of Vale do Taquari—UNIVATES, to assess the potential for energy generation after the end of each seasonal period. The samples from the tank containing the macrophytes used in the VFCW were collected after pruning, carried out in two periods: one after the winter and spring 2019 and another after the summer and autumn 2020, making a 6-month interval between the samplings (Fig. 2B).
The methodological difference between the collection of microalgae biomass and the macrophyte biomass of the VFCW is mainly due to the developmental characteristics of plants (slower growth cycle) and microalgae (faster growth cycle). The collection of microalgal biomass was carried out in two stages: in the first, after 7 days HRT, the biomass was removed after passing the pre-treated wastewaters through the microalgae by the biomass separating filter (Fig. 3C) and reintroduced into the photobioreactor (Fig. 3B). In a second moment, after 14 days (HRT), the biomass was again removed after passing through the separator filter and together with the biomass adhered to the photobioreactor and the box, collected, preserved, and refrigerated.
The analyses were performed by an automated biogas flow measurement system through a programmable logic controller (PLC) system called automated biogas measurement system (ABMS) and with the automatic methane potential test system (AMPTS II) as described by Konrad et al. 2022).
Experiment and inoculum characterization
Biogas analyses were performed by an automated biogas flow measurement system through a programmable logic controller (PLC) system called automated biogas measurement system (ABMS) and with the automatic methane potential test system (AMPTS II) as described by Konrad et al. (2022)
The inoculum used for anaerobic digestion of the respective biomass was provided by the Research Center in Energy and Sustainable Technologies (CPETS) of UNIVATES, which comes from previous experiments already carried out, thus containing microorganisms fully adapted to mesophilic anaerobic digestion (37 °C). The inoculum used presented basic characteristics with precision and efficiency, among them the pH > 7.0 and < 8.5, ammonia < 2.5 g of N-NH4 L−1, and alkalinity > 3 g CaCO3 L−1.
The inoculum/sample ratio was defined according to the protocol applied in the experiment, based on the calculation of total solids (TS) and volatile solids of the sample (VSS), as described in the Standard Methods (2017), and volatile solids of the inoculum (VSI) using a 2 × 1 ratio (VDI 4630 2006). The VDI 4630 method uses the values of the total and volatile solids of the inoculum and the substrate evaluated for the purpose of proportion in the percentages present in each plot in the experiment or that will be part of the experiment to be tested.
Anaerobic digestion assay
The VDI 4630 (2006) method was used to carry out the anaerobic digestion in this experiment. The method primarily consists of determining the inoculum solids and the biomass to be tested, as described above. pH analyses were also performed. For this purpose, homogenized portions of these two materials were separated, proceeding with the analyses and storing the remainder for the time of testing effectively, with the inoculum being kept in the incubator and the biomass in the refrigerator.
The biomass inoculated in the reactor was weighed (Fig. 4A) and then the inoculum/sample ratio was defined. In the reactors, previously defined sample and inoculum were added. The exact amount added to each reactor, both inoculum and sample, was duly registered through a written note (Fig. 4B, C). Thereafter, the material was homogenized and the pH analyses of each reactor were performed.
Methane content evaluation
The methodology adopted for monitoring and measuring the generated biogas was similar to the described by Konrad et al. (2016b). The proposed system uses liquid displacement as the measurement principle. Methane was analyzed by the advanced Gasterter specific sensor, produced by PRONOVA Analysentechnik GmbH & Co.
Data and statistical analysis
The obtained results were subjected to statistical analysis using the software GraphPad Prism 8.0. (GraphPad Inc., CA, USA). Total P, COD, BOD5, N-NH3, TSD, turbidity, and conductivity as well as the biogas production considering different seasons were subjected to one-way ANOVA analysis. To detect the statistical significance of differences (p < 0.05) among raw wastewaters and the different units of the hybrid system, Tukey’s multiple comparison test was performed.
Results and discussion
Microalgal native species from the southern region of Brazil, where the pilot project was developed and operated, were utilized for biomass production As it is an open system, after the acclimatization period, the photobioreactor developed a green color indicating the presence of microalgae mostly of the Chlorophycea class (Fig. 5A). The plant used in VFCW was the emerging macrophyte Hymenacne grumosa, a native species of the study region, abundant in the southern fields and widely used as animal food. The option for this native species is due to its high biomass production and its capability of achieve good results regarding the reduction of physicochemical parameters as described by Silveira et al. (2019).
The biomass production related to the macrophyte used in the VFCW was similar to that described by Silveira and Machado (2018), who carried out a study using the species H. grumosa as a phytoremediator of university campus wastewaters. The biomass generated was originated from the treatment of university campus wastewaters treated during the study period. The results referring to physicochemical monitoring show a reduction of 61% for total phosphorus, 71.7% for COD, 39.9% for BOD5, 98.9% for ammoniacal nitrogen, 92.1% for turbidity, and 90% for apparent color removal compared to the data recorded for the raw wastewaters (Table 1). In addition, the integrated system achieved disinfection rates of 99.99% for thermotolerant coliforms and Escherichia coli (Table 1), an index similar to those described by Silveira et al. (2020), who developed a study with an integrated system composed of sequential microalgae and CWs and focused on evaluating the detoxification potential by comparing raw and treated wastewaters.
In relation to the results of the biogas production from the analyzed biomass, it was possible to observe a difference between the analyzed stages (Table 2), although these differences were already expected, due to the structure of the experiment, which was configured to operate in an “open” way, that is, fully exposed to natural weather and without control of environmental variables.
Regarding the biogas generation during the research period, it was possible to verify that the highest production rates involving microalgae were attained during the spring season. On the other hand, values significant lower were measured in winter 2019, summer 2020, and autumn 2020 (p < 0.0001). When considering the constructed wetland unit, significant higher biogas values were produced by H. grumosa in winter and spring 2019 in comparison to summer and autumn 2020 (p < 0.0001). It must be pointed out that these differences were enhanced between the experiments carried out in 2019 (winter and spring) and 2020 (summer and autumn), probably because they were extremely affected by two main factors: the dry period that severely hit the study region (December/2019 to May/2020) and the drastic reduction in the generation of university wastewaters because of the university recess period (holidays) and the COVID-19 global pandemic. These factors hampered the supply of the integrated system, since, in addition to suffering from the drought, the wastewaters generated during the period were far below standards, both in terms of quantity and organic load. So, the performance and energy generation of the integrated system was affected, consequently interfering in the energy production of biomass generated in the period.
During the collection and analyses stages of samples corresponding to winter 2019 and spring 2019 (Table 2), it was possible to observe that the averages of CH4 generation in this study obtained similar results to those found in other bioenergy production studies with different substrates, as, for example, the results described by Silva et al. (2020) that obtained CH4 generation of 60% on average using municipal solid waste as biomass.
In the studies carried out by Lin et al. (2011), using fruit residues and food waste, and Zhang et al. (2013) who used food waste and cattle manure, respectively, the concentration of CH4 presented an average of 58.75% and 41%, respectively. The means obtained in the present study in the winter and spring stages registered CH4 values for microalgal biomass, equivalent to those found by Lin et al. (2011), while the results obtained with biomass from plants in the VFCW (H. grumosa) were similar to those found by Zhang et al. (2013).
Alzate et al. (2012) researched the potential for biogas and methane generation of different genera of algae and microalgae, with results of biochemical biogas potential (BBP) and biochemical methane potential (BMP) for Chlorophycea microalgae of 581.08 NmL-gSV−1 and 398 Nml-gSV−1, respectively. Values very similar to those described in this study, where data of 502.70 NmL-gSV−1 and 312.43 NmL-gSV−1 were recorded (Fig. 6A, B), during the spring period, the main period of microalgae development.
Keymer et al. (2013), when using high pressure thermal hydrolysis (HPTH) to verify the methane yield potential during the digestion of microalgal biomass in the biogas generation process, found BBP and BMP values for Chlorophycea of 798 NmL-gSV−1 and 307 Nml-gSV−1, similar to those showed in Fig. 6A.
Studies carried out by Wöhler-Geske et al. (2016), using the VDI 4630 (2006) method as a reference, analyzed different reed species obtained from sugarcane cutters in Germany, the Netherlands, and other reed exporting countries around the world associated with the generation of biogas. The results indicated values with BBP of 551.5 NmL-gSV−1 and BMP of 311.4 NmL-gSV−1, very close to the values obtained in the present research, mainly in step I (first pruning—macrophyte), where the observed BBP and BMP values were of 554.49 Nml-gSV−1 and 312.26 Nml-gSV−1 respectively.
Roj-Rojewski et al. (2019) examined the biogas and methane production of reed species of the genera Phragmites, Glyceria, and Carex, harvested in the Narew River valley in northeastern Poland. The authors found levels of BMP 102–221 Nml-gSV−1, similar to those observed for grasses collected in fields and natural wetlands by other researchers. However, these values were much lower when in relation to species obtained from pastures or CWs.
The results described by Roj-Rojewski et al. (2019) point out as a reason for this difference in energy capacity, the suboptimal condition of the C: N ratio. This fact may explain the results obtained in the experiment represented in Fig. 6C, D (second stage) in which the data presented 258.1 Nml-gSV−1 and 50.50 Nml-gSV−1 of PBB and PBM, respectively. Although both species of plants and the environmental conditions were different, there is a possibility that the remarks described by Roj-Rojewski et al. (2019) might elucidate the cause related to low yield in biogas generation during step 2 of the analyses of this study.
In the case of the aforementioned authors, the plants used were obtained from a preserved environment, since the Narew River valley is located in a national reserve in Poland. This fact initially refers to a place without pollution, therefore without an abundance of nutrients dissolved in water, place where plants grow. In the present study, the step of biomass obtainment for the plants used in the VFCW (second pruning—macrophyte) was carried out during the pandemic period, therefore, without activity on the university campus and, consequently, without the generation of wastewaters at the WWTP.
It is possible that the results referring to analyses equivalent to the periods of summer and autumn/2020 (Fig. 6A, B) were affected by the same factor described by Roj-Rojewski et al. (2019), since the microalgae used, despite having a less complex cellular composition than the macrophytes, also suffered from the lack of nutrients contained in the university effluent due to the COVID-19 pandemic, unbalancing the C to N ratio.
In Table 3, we can see some studies that used biogas to generate electricity, using different substrates, as a basis for comparison with the results obtained by the experiment with the biomass generated by the microalgae photobioreactor (present work).
The production of electric energy through the biomass generated by microalgae during the spring period demonstrates that the generation values obtained were similar to the results described by the authors represented in Table 3.
Mello et al. (2018) described an electric energy production index from biogas of 1.44 kWh/m3, while the present work presented a generation potential of 1.50 kWh/m3. The data presented in Table 3 demonstrate that the potential electricity generation from the conversion of microalgae biomass into biogas were similar to those described by the cited studies, a fact that may be encouraging in terms of the use of this biomass as a source of energy production.
The results presented in able 3 were used as a basis for comparison with the obtained in the present work. However, the values found by other researchers referring to the conversion of biogas into electrical energy may present discrepancies due to the different equipment used in each study. Even so, the results described are within acceptable values, close to those found in our study. Dalpaz et al. (2020) obtained results regarding energy generation of 2.04 kWh/m3 considering 68% of CH4 content from agro-industrial residues. The cited study presented the highest energy among the works described in Table 3, possibly due to the high organic matter concentration, thus increasing the calorific power of the used biomass. Also, the ratio of electrical energy generation in relation to thermal energy can fluctuate due to the differences between the equipment that performs this type of experiment. According to the literature, thermal energy can reach two thirds of the electrical energy (Dalpaz et al. 2020). On the other hand, the biomass used in this study presented potential electric generation of 1.50 kWh/m3 based on 58% of CH4, which can generate an equivalent of 4.50 kcal/kg of thermal energy.
The results described in this study reinforce the idea of integrating actions aimed at the application of nature-based solutions (NBS), from phytoremediation processes, since the conversion of biomass into energy was part of a process created to detoxify urban wastewaters, thus being an initiative to develop treatments based on self-sustainable cycles applied to environmental sanitation.
Conclusions
The levels of energy generation obtained in this study showed encouraging results, especially considering the analysis stage referring to the spring period, where the most favorable climatic conditions for microalgae development and proliferation occur. The results obtained demonstrated that both microalgal biomass and macrophyte biomass can be used as raw material for the production of bioenergy, being an excellent environmental alternative. Thus, the conversion of biomass, which would be a potential waste, into thermal energy, demonstrates that it is possible to develop ideas that can bring combined environmental solutions, enhancing environmental sanitation projects as applied sustainable alternatives.
The results obtained showed a promising electric energy generation potential, when compared with other studies, reinforcing the idea of using the biomass generated during the effluent treatment process.
Finally, we can prospect future advances using the proposed urban effluent treatment technology with a real-scale integrated system for decentralized treatment and energy recovery using different native species from diverse regions. Still, we can indicate the use of the Life Cycle Assessment (LCA) tool as a future work to scientifically demonstrate the feasibility of developing the proposed technology.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abad AV, Cherrett T, Holdsworth P (2015) Waste-to-fuel opportunities for British quick service restaurants: a case study. Resour Conserv Recycl 104:239–253
Alzate ME, Muñoz R, Rogalla F, Fdz-Polanco F, Pérez-Elvira SI (2012) Biochemical methane potential of microalgae: influence of substrate to inoculum ratio, biomass concentration and pretreatment. Biores Technol 123:488–494
AOAC – Association of Official analytical Chemists (2000) Method 990. n. 12 Official methods of analysis of AOAC International. 17. ed. Gaithersburg: AOAC International, p 22–23
APHA/AWWA – American Public Health Association (2012) Standard methods for the examination of water and wastewater. 21. ed. Washington: APHA/AWWA/WEF
Coimbra-Araújo CH, Mariane L, Júnior CB, Frigo EP, Frigo MS, Araújo IRC, Alves HJ (2014) Brazilian case study for biogas energy: production of electric power, heat and automotive energy in condominiums of agroenergy. Renew Sustain Energy Rev 40:826–839
Ardolino F, Arena U (2019) Biowaste-to-Biomethane: an LCA study on biogas and syngas roads. Waste Manag 2019(87):441–453
Ardolino F, Cardamone GF, Parrillo F, Arena U (2021) Biogas-to-biomethane upgrading: a comparative review and assessment in a life cycle perspective. Renew Sustain Energy Rev 139:110588
Callegari A, Bolognesi S, Cecconet D, Capodaglio AG (2020) Production technologies, current role, and future prospects of biofuels feedstocks: a state-of-the-art review. Crit Rev Environ Sci Technol 50(4):384–436
Capodaglio AG, Bolognesi S, Cecconet D (2021) Sustainable, decentralized sanitation and reuse with hybrid nature-based systems. Water 13(11):1583
Capodaglio AG, Callegari A, Lopez MV (2016a) European framework for the diffusion of biogas uses: emerging technologies, acceptance, incentive strategies, and institutional-regulatory support. Sustainability 8(4):298
Capodaglio AG, Ranieri E, Torretta V (2016b) Process enhancement for maximization of methane production in codigestion biogas plants. Manag Environ Qual Int Journal 27(3):289–298
CIBIOGÁS (2021) Nota Técnica: N° 001/2021 – Panorama do Biogás no Brasil 2020. Foz do Iguaçu: CIBiogás
Dalpaz R, Konrad O, da Silva Cyrne CC, Barzotto HP, Hasan C, Guerini Filho M (2020) Using biogas for energy cogeneration: an analysis of electric and thermal energy generation from agro-industrial waste. Sustainable Energy Technol Assess 40:100774
Da Ros C, Cavinato C, Pavan P, Bolzonella D (2014) Winery waste recycling through anaerobic co-digestion with waste activated sludge. Waste Manage 34(11):2028–2035
Da Silva RC, de Marchi Neto I, Seifert SS (2016) Electricity supply security and the future role of renewable energy sources in Brazil. Renew Sustain Energy Rev 59:328–341
Dos Santos EB, de Nardi GJR (2013) Produção de biogás a partir de dejetos de origem animal. Tekhne e Logos 4(2):80–90
González-Fernández C, Sialve B, Bernet N, Steyer JP (2012) Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Bioenerg 40:105–111
Gutiérrez R, Passos F, Ferrer I, Uggetti E, García J (2015) Harvesting microalgae from wastewater treatment systems with natural flocculants: effect on biomass settling and biogas production. Algal Res 9:204–211
Hahn H, Krautkremer B, Hartmann K, Wachendorf M (2014) Review of concepts for a demand-driven biogas supply for flexible power generation. Renew Sustain Energy Rev 29:383–393
Hasan C, Marder M, Hickmann EV, Feldkircher T, Bücker F, Konrad O (2018) Biogas generation related to carbon removal from anaerobic co-digestion of sludge, blood, and swine manure combined in different proportions: production of biogas by anaerobic co-digestion. Environ Qual Manage 28(1):115–122
INSTITUTO 17 (2021) Biogás no Brasil: Potencial Oferta a Curto Prazo. São Paulo/SP: Programa de Energia para o Brasil – BEP (Brasil)
Karlsson T (2014) Manual básico de biogás / Tommy Karlsson [et al]. - Lajeado: Ed. da Univates, p 69
Keymer P, Ruffell I, Pratt S, Lant P (2013) High pressure thermal hydrolysis as pre-treatment to increase the methane yield during anaerobic digestion of microalgae. Biores Technol 131:128–133
Konrad O, Hasan C, Marder M, Zulian L, Guerini Filho M (2022) Comparison of two gas volume measurement systems by evaluating biochemical methane potential. Environ Qual Manage 31(3):201–207
Konrad O, Bezama AB, Prade T, Backes GM, Oechsner H (2016a) Enhancing the analytical capacity for biogas development in Brazil: assessment of an original measurement system for low biogas flow rates out of agricultural biomass residues. Engenharia Agrícola 36:792–798
Konrad O, Akwa JV, Koch FF, Lumi M, Tonetto J (2016b) Quantification and characterization of the production of biogas from blends of agro-industrial wastes in a large-scale demonstration plant. Acta Scientiarum Technology 38(4):415–421
Lantz M (2012) The economic performance of combined heat and power from biogas produced from manure in Sweden–a comparison of different CHP technologies. Appl Energy 98:502–511
León E, Martín M (2016) Optimal production of power in a combined cycle from manure-based biogas. Energy Convers Manage 114:89–99
Lin J, Zuo J, Gan L, Li P, Liu F, Wang K, ..., Gan H (2011) Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J Environ Sci 23(8):1403-1408
Martens M, Karlsson NP, Ehde PM, Mattsson M, Weisner SE (2021) The greenhouse gas emission effects of rewetting drained peatlands and growing wetland plants for biogas fuel production. J Environ Manage 277:111391
Martinez DG, Kitamura DS, Silva FP, Souza SMN, Bastos RK, Souza CRB (2013) Geração de energia elétrica a partir do biogás. Brazilian J Biosyst Eng 7:45–54. https://doi.org/10.18011/bioeng2013v7n1p45-54
Mello VM, Santos DDL, Freitas RCS, Yokoyama L, Cammarota C (2018) Energy generation in the treatment of effluent from washing of municipal solid waste collection trucks. Sustainable Energy Technol Assess 30:105–113. https://doi.org/10.1016/j.seta.2018.09.009
Pacheco MM, Hoeltz M, Moraes MS, Schneider RC (2015) Microalgae: cultivation techniques and wastewater phycoremediation. J Environ Sci Health A 50(6):585–601
Passos F, Astals S, Ferrer I (2014) Anaerobic digestion of microalgal biomass after ultrasound pretreatment. Waste Manage 34(11):2098–2103
Prochnow A, Heiermann M, Drenckhan A, Schelle H (2005) Seasonal pattern of biomethanisation of grass from landscape management. Agricultural Engineering International: The CIGR Journal 7
Raslavičius L, Grzybek A, Dubrovin V (2011) Bioenergy in Ukraine—possibilities of rural development and opportunities for local communities. Energy Policy 39(6):3370–3379
Ribeiro EM, Barros RM, Tiago Filho GL, dos Santos IFS, Sampaio LC, dos Santos TV, ..., de Freitas JVR (2016) Power generation potential in posture aviaries in Brazil in the context of a circular economy. Sustain Energy Technol Assess 18:153-163
Roj-Rojewski S, Wysocka-Czubaszek A, Czubaszek R, Kamocki A, Banaszuk P (2019) Anaerobic digestion of wetland biomass from conservation management for biogas production. Biomass Bioenerg 122:126–132
Ryckebosch E, Drouillon M, Vervaeren H (2011) Techniques for transformation of biogas to biomethane. Biomass Bioenerg 35(5):1633–1645
Silva CO, Konrad O, Callado NH, Marder M, de Araujo LGS (2020) Resíduos sólidos orgânicos domésticos como substrato potencial para produção de biogás. Revista Ibero-Americana De Ciências Ambientais 11(2):204–212
Silveira EO, Lutterbeck CA, Machado EL, Rodrigues LR, Rieger A, Beckenkamp F, Lobo EA (2020) Biomonitoring of urban wastewaters treated by an integrated system combining microalgae and constructed wetlands. Sci Total Environ 705:135864
Silveira EO, Moura D, Rieger A, Machado ÊL, Lutterbeck CA (2017) Performance of an integrated system combining microalgae and vertical flow constructed wetlands for urban wastewater treatment. Environ Sci Pollut Res 24(25):20469–20478
Silveira EO, Wink M, Zappe AL, Kist LT, Machado ÊL (2019) Sistema integrado com microalgas e wetland construído de fluxo vertical no tratamento de efluentes urbanos. Engenharia Sanitaria e Ambiental 24:305–313
Silveira EO, Machado ÊL (2018) Fitorremediação de Efluentes Urbanos: Microalgas e Wetlands Construídos: Saneamento Ambiental Como Tecnologia Mais Limpa. Novas Edições Acadêmicas,2018.
Standard Methods (2017) Standard methods for the examination of water and wastewater, 2540 Solids- 2540 G. Total, Fixed, and Volatile Solids in Solid and Semisolid Samples 23th Edition, 2017.
VDI V (2006) Richtlinie 4630: Vergärung organischer Stoffe Substratcharakterisierung, Probenahme, Stoffdatenerhebung, Gärversuche. Verein Deutscher Ingenieure (Hrsg.): VDI-Richtlinie 4630
Wall DM, Allen E, O’Shea R, O’Kiely P, Murphy JD (2016) Investigating two-phase digestion of grass silage for demand-driven biogas applications: Effect of particle size and rumen fluid addition. Renew Energy 86:1215–1223
Whiting A, Azapagic A (2014) Life cycle environmental impacts of generating electricity and heat from biogas produced by anaerobic digestion. Energy 70:181–193
Wöhler-Geske A, Moschner CR, Gellerich A, Militz H, Greef JM, Hartung E (2016) Yield, fermentation kinetics and the role of quality properties of thatching reed (Phragmites australis) during discontinuous anaerobic fermentation. Ind Crops Prod 83:701–709
Wu W, Chang J (2019) Integrated algal biorefineries from process systems engineering aspects: a review. Bioresource Technology 291. https://doi.org/10.1016/j.biortech.2019.121939
Zhang C, Xiao G, Peng L, Su H, Tan T (2013) The anaerobic co-digestion of food waste and cattle manure. Biores Technol 129:170–176
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development—CNPq of Brazil (141416/2018-1).
Author information
Authors and Affiliations
Contributions
Elizandro Oliveira Silveira: methodology—development of methodology, investigation—conducting research, writing—original draft.
Nathalia Mendes Felizzola: methodology—development of methodology.
Eugênia Vargas Hickmann: investigation—performing experiments and data collection.
Odorico Konrad: investigation—performing experiments and data collection.
Carlos Alexandre Lutterbeck: writing—reviewing and editing and statistical analysis.
Ênio Leandro Machado: conception of the treatment system, writing—review and editing.
Lucia Ribeiro Rodrigues: advisor, conception of the treatment system, writing—review and editing, supervision.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Alexandros Stefanakis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silveira, E.O., Felizzola, N.M., Hickmann, E.V. et al. Energy recovery by anaerobic digestion of algal biomass from integrated microalgae/constructed wetland wastewater treatment. Environ Sci Pollut Res 30, 13317–13326 (2023). https://doi.org/10.1007/s11356-022-23019-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23019-3