Abstract
Cyclophosphamide (Cyclo) is a chemotherapeutic agent used as an immunosuppressant and as a treatment for many cancerous diseases. Many previous pieces of literature proved the marked cardio and neurotoxicity of the drug. Thus, this research provides evidence on the alleviative effect of flavocoxid on the cardiac and brain toxicity of cyclophosphamide in mice and determines its underlying mechanisms. Flavocoxid (Flavo) is a potent antioxidant and anti-inflammatory agent that inhibits the peroxidase activity of cyclooxygenase (COX-1 and COX-2) enzymes and 5-lipooxygenase (5-LOX). Flavo was administered orally (20 mg/kg) for 2 weeks, followed by Cyclo (100 mg/kg, i.p.) on day 14. Higher heart and brain weight indices, serum lactate dehydrogenase (LDH), creatine kinase (CK-MB), and nitric oxide (NO) were mitigated following Flavo administration. Flavo modulated oxidative stress biomarkers (malonaldehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD)), tumor necrosis factor-α (TNF-α), and interleukin (IL)-1β. Additionally, cardiac troponin I (cTn-I), nuclear factor kappa B (NF-κB), brain amyloid precursor protein (APP), and granulocyte macrophage colony-stimulating factor (GM-CSF) were decreased by Flavo administration. Moreover, Flavo ameliorated heart and brain histopathological changes and caspase-3 levels. Collectively, Flavo (20 mg/kg) for 14 days showed significant cardio and neuroprotective effects due to its antioxidant, anti-inflammatory, and antiapoptotic activities via modulation of oxidative stress, inflammation, and the GM-CSF/NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclophosphamide (Cyclo) is a potent alkylating cytotoxic agent used as a treatment for various types of cancer. Additionally, it is a potent immunosuppressant used for organ transplantation and autoimmune diseases (Chen et al. 2017; Elmariah et al. 2018). Cyclo exerts its antineoplastic cytotoxic effects through its active metabolite, phosphoramide. At the same time, acrolein is the metabolite that is mainly responsible for Cyclo-induced toxicities, including cardiotoxicity, nephrotoxicity, hepatotoxicity, male reproductive toxicity, teratogenicity, and neurotoxicity (Avci et al. 2017; Zhai et al. 2018). Clinically, Cyclo-induced cardiotoxicity is dose-dependent and is characterized by acute onset (Goldberg et al. 1986; Braverman et al. 1991). Cyclo has also been reported to cause brain toxicity in animal studies, but clinically, it has not been well estimated (Chen et al. 2019). Furthermore, several studies have showed a massive elevation of reactive oxygen species (ROS) after Cyclo administration. This increase in ROS damages cellular biomolecules, leading to cell death (Aladaileh et al. 2019). Additionally, Cyclo can induce toxicity through the depletion of cellular decreased glutathione (GSH) and superoxide dismutase (SOD), thus leading to the upregulation of MDA and increased nitric oxide (NO), resulting in multiorgan hazardous side effects due to oxidative stress (Moghe et al. 2015; Aladaileh et al. 2019). Moreover, Cyclo can form a protein adduct in cardiomyocytes through its toxic metabolite acrolein, leading to NF-κB activation, the main inducer of proinflammatory gene induction, and functions. After translocation to the nucleus, activated NF-κB transcript the production of inflammatory cytokines, such as interleukin 1β (IL-1β) and tumor necrosis factor-α (TNF-α), elevating their levels (Iqubal et al. 2019a). However, some studies showed that Cyclo could cause marked neurotoxicity (Rzeski et al. 2004; Seo et al. 2019). Cyclo can cause a significant oxidative imbalance in the brain, which can be lowered by the coadministration of free radical scavengers (Singh and Kumar 2019). Thus, in the current research, Flavo is used as an antioxidant agent with Cyclo to reduce its toxicity.
Flavocoxid (Flavo) is a medical food consisting of purified plant-based bioflavonoids, including baicalin, extracted from Scutellaria baicalensis, and catechin, from Acacia catechu, with a concentration higher than 90% purity (Burnett et al. 2011). Flavo causes balanced inhibition of both cyclooxygenase-1 (COX-1) and COX-2 peroxidase enzyme activity and shows significant potent inhibition of 5-lipooxygenase (5-LOX) (Altavilla et al. 2009; Burnett et al. 2011). Additionally, it has anti-inflammatory and immunomodulatory activities and is a potent free radical scavenger (Messina et al. 2009; Polito et al. 2010; Bitto et al. 2012). Flavo downregulates inflammatory markers not only at the protein level but also at the gene expression level. Moreover, Flavo can lower oxidative imbalance by inhibiting NF-κB, the primary mediator of proinflammatory gene induction, and functions. As a result, Flavo can downregulate the gene expression of COX-2, 5-LOX, iNOS, and TNF-α, limiting their levels and their metabolites, including prostaglandin E2 (PG-2), leukotriene B4 (LTB4), and NO (Altavilla et al. 2009; Burnett et al. 2011).
The current study was conducted to assess the potential beneficial effect of flavocoxid, a selective COX-1/COX-2 peroxidase inhibitor, on the progression of cardio and neuroinjury and its associated pathological and biochemical consequences in an experimental model of cyclophosphamide-induced cardio and neurotoxicity in mice.
Materials and methods
Chemicals and treatments
Cyclo was bought from Astra medica co. Egypt as “Endoxan (1 g)” and was diluted with distilled water and injected intraperitoneally. Flavo was bought from MEDVANTX, USA, as “Limbrel 250™” and was suspended in carboxymethyl cellulose (CMC, El-Nasr co. Egypt) and taken orally. Thiopental sodium, acetic acid, AA (20%), KCL, and thiobarbituric acid, Na dodecylsulfate, pyrogallol, tris buffer, phosphate buffer, TCA-EDTA, and Elman’s reagent were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
Animals
Forty male Swiss albino mice weighing approximately 20–25 g were purchased from the “Egyptian Organization for Biological Products and Vaccines,” Giza, Egypt. They were kept in standard animal care efficiency (animal house, Faculty of Pharmacy, Mansoura University, Egypt). Experimental procedures were approved by the “Research Ethics Committee,” Faculty of Pharmacy, University of Mansoura, Egypt.
Study design
One week passed on acclimation of the mice, and then, they were divided into four groups (n = 10 mice).
-
(1)
The control group was provided with the solvent (0.5% CMC) from day 0 to day 14.
-
(2)
Administration of Flavo (20 mg/kg) orally for 2 weeks.
-
(3)
The Cyclo group was injected with Cyclo (100 mg/kg, i.p.) as a single dose on day 14.
-
(4)
The protected group was given Flavo from day 0 to day 14 with Cyclo (100 mg/kg) as a single dose on day 14 (Abdelaziz et al. 2018).
Blood sampling
Before sacrificing, blood samples were collected from the retro-orbital sinus, and sera were separated and stored at − 80 °C to estimate LDH, CK-MB, and NO.
Heart and brain weight/body weight ratio determination and heart and brain homogenate preparation
Mice were sacrificed under mild thiopental anesthesia (50 mg/kg, i.p.), and then, whole hearts and brains were removed, cleaned with ice-cold 1.15% potassium chloride (KCl) at pH 7.45, and scaled. Then, the heart and brain weight per body weight ratio was measured. Chilled KCl was applied to the first portion of the heart and brain tissue for homogenization using a glass homogenizer on ice to prepare 10% w/v tissue homogenates. After that, the homogenate was centrifuged at 4000 rpm and 4 °C; the supernatant was detached and finally isolated into aliquots and frozen at − 80 °C (Maresh et al. 2020).
Estimation of serum biochemical parameters
Lactate dehydrogenase
LDH was determined quantitatively following the method of Henry (1974). LDH is responsible for the conversion of pyruvate to L-lactate oxidizing NADH, which absorbs light at 340 nm. NADH oxidation is directly proportional to the catalytic activity of LDH.
Creatinine kinase
CK-MB was quantitatively assessed following the method of Wurzburg et al. (1977). The antibody inhibits subunit M of CK-MB. The noninhibited CK-MB undergoes a series of reactions, producing NADPH, which is measured photometrically. CK-MB was calculated as unit per liter (Würzburg et al. 1977).
Nitric oxide
In an acidic medium and in the presence of nitrite, one of the final two nitric oxide products, the formed nitrous acid diazotizes sulfanilamide, and the product was complex with V- (naphthyl) ethylene diamine. The resulting bright reddish-purple azo dye can be measured at 540 nm (Amin et al. 2020).
Estimation of brain and heart oxidative stress
Heart and brain malonaldehyde level
MDA content was estimated using the method of Ohkawa et al. (1979). Thiobarbituric acid reactive substances were estimated as MDA. MDA was expressed as nanomole/gram wet tissue at 532 nm.
Heart and brain glutathione level
GSH level was assessed using the method of Ellman (1959). GSH was expressed as micromole/gram wet tissue at 412 nm.
Heart and brain superoxide dismutase level
SOD activity was determined using the method of Marklund and Marklund (1974). SOD activity was expressed as U/gram tissue at 420 nm.
Estimation of heart and brain tumor necrosis factor-α and interleukin-1β
The TNF-α and IL-1β kits are sandwich enzyme immunoassay for in vitro quantitative measurement of TNF-α and IL-1β (eBioscience, USA).
Estimation of cardiac troponin I and brain granulocyte macrophage-colony stimulating factor in heart and brain homogenates
cTn-I and GM-CSF levels were determined using a double-antibody sandwich ELISA kit procured from Biocodon Technologies, USA.
Western blot analysis
The expression levels of nuclear factor kappa B (NF-κB) and amyloid precursor protein (APP) in heart and brain tissues, respectively, were evaluated using western blot analysis. A Bradford Protein Assay Kit (SK3041; Markham Ontario L3R 8T4 Canada) was used to run the protein concentration assay. A total of 20 µg of protein in each sample was loaded and separated using electrophoresis on a polyacrylamide gel (TGX Stain-Free™ FastCast™ Acrylamide Kit (SDS-PAGE) and then transferred onto a PVDF membrane. The membrane was blocked with Tween 20 (TBST) buffer and 3% bovine serum albumin (BSA) at 25 °C for 1 h. Primary antibodies of APP and NF-κB were bought. Incubation was performed overnight in each primary antibody solution against the blotted target protein at 4 °C (Cell Signaling Technology). The blot was then washed and incubated with HRP-conjugated secondary antibody (goat antirabbit IgG-HRP-1 mg Goat mab-Novus Biologicals). A chemiluminescence substrate was detected by antibody interaction (ClarityTM Western ECL substrate Bio-Rad cat#170–5060). A CCD camera–based imager was used to capture the chemiluminescent signals. Software of image analysis was used to read the band intensity of the target proteins against control sample beta actin (housekeeping protein) by protein normalization on the ChemiDoc MP imager.
Histopathological examination
A small second portion of heart and brain tissues of mice were histopathologically figured out. Samples were settled with 10% formalin buffer for 24 h at 25 °C before they were fixed in paraffin and sliced (3–5 µm). Finally, H&E staining was performed, and the slides were seen in a blind manner (Maresh et al. 2020).
Immunohistochemical examination of cardio and neuroapoptosis (caspase-3)
Immunohistochemical examination was applied for a sensitive marker for apoptosis (caspase-3) following the manufacturer’s instructions (Biocare Medical, Pacheco, CA, USA). The paraffin was segregated from the paraffin-embedded third portion of the heart and brain sections before the endogenous peroxidase activity was frustrated with 3% H2O2. Citrate buffer used in antigen retrieval achievement. Then, 5% BSA was conducted on the heart and brain sections to remove any nonspecifically bound protein after cooling. Then, the sections were brooded overnight (4 °C) with primary antibody (rabbit monoclonal anticaspase-3). PBS cleaned the sections, afterward; they were incubated with a secondary conjugated polymer for 30 min at RT on the next day. The next step was incubation for 5 min RT with Biocare’s 3,3-diaminobenzidine (DAB) or for 6–8 min at 25 °C with Biocare’s Warp Red Counterstain with Mayer’s hematoxylin, followed by soaking with deionized water. Tacha’s Bluing Solution was performed for 1 min, followed by wetting with deionized water. Biocare’s MACH 4 detection system was employed for standardizing this antibody. Pictures of sections were taken under a microscope (Nikon) (Gown and Willingham 2002).
Statistical analysis
Data (means ± SEM) are shown. Statistical graphing and analysis were conducted using GraphPad Prism 6 software (CA, USA). The significance level was set at p < 0.05. The statistical difference in the results was determined using one-way analysis of variance (ANOVA), followed by Tukey–Kramer test. The study of immunohistochemistry scores was conducted using a nonparametric Kruskal–Wallis test, followed by Dunn’s multiple comparison post hoc test.
Results
Influence of Flavo on heart and brain indices in Cyclo-injected mice
There was no significant difference in heart weight index between all groups, and in the brain, Cyclo increased its weight index by 22.22% and 33.33% compared to the control and Flavo groups, respectively. Flavo in treated group reduced the brain weight index by 11.11% compared to the Cyclo group (Table 1).
Influence of Flavo on serum biochemical parameters (LDH, CK-MB, and NO) in Cyclo-injected mice
After the administration of Cyclo (100 mg/kg, i.p.) injection, there were significant increases in LDH, CK-MB, and NO by 228%, 113%, and 85%, respectively, compared with those in the control group. However, serum LDH, CK-MB, and NO in the protected group significantly decreased by 59%, 59%, and 61%, respectively, compared with the Cyclo-treated group. Oral Flavo alone caused no significant difference in the measured biochemical parameters compared with those of the control group (Table 2).
Influence of Flavo on heart and brain oxidative stress biomarkers in Cyclo-injected mice
-
a.
Influence on MDA
Cyclo significantly increased MDA levels in heart homogenates by 48% and 42.2% compared to the control and Flavo groups, respectively, and in brain homogenates by 30% and 49.7% compared to the control and Flavo groups. On the other side, Flavo in the protected group decreased MDA level by 38% and 37% in the heart and brain, respectively, compared to the Cyclo group (Fig. 1A).
-
b.
Influence on GSH
Cyclo markedly depleted the GSH level in heart homogenates by 79% and 103% compared to the control and Flavo groups, respectively. However, Flavo increased GSH levels in the heart by 85% compared to the Cyclo group, and there is no significant difference in brain GSH compared to the Cyclo group (Fig. 1B).
-
c.
Influence on SOD
Cyclo decreased heart SOD level significantly by 461% and 471% compared to the control and Flavo groups, respectively, and in brain homogenates similarly by 27% and 43%. Flavo increased its level markedly by 479.3% and 90% in heart and brain homogenates, respectively, compared to the diseased group (Fig. 1C).
Effect of Flavo on oxidative stress biomarkers in Cyclo-injected mice. Oral administration of (20 mg/kg) flavocoxid (Flavo) to mice from day 0 to 14 followed by cyclophosphamide (Cyclo) intraperitoneal (100 mg/kg) on day 14. Data were expressed as mean ± SED (n = 10). *, $, #Significantly different compared to normal control, Flavo, and the Cyclo groups, respectively, using one-way ANOVA test with Tukey–Kramer post hoc test (p < 0.05). (A) MDA, (B) GSH, and (C) SOD
Influence of Flavo on heart and brain TNF-α and IL-1β in Cyclo-injected mice
Cyclo significantly elevated TNF-α in heart and brain homogenates by 4.25- and 2.6-fold, respectively, compared with the healthy control group. However, Flavo in the protected group decreased TNF-α level by 69% and 45% in heart and brain homogenates, respectively, compared to the diseased group (Fig. 2A). Regarding IL-1β, Cyclo markedly increased its level in heart homogenates by 79.7% and 82% compared with the control and Flavo groups, respectively, and similarly in the brain by 80% and 78.6%. In the Flavo group, there was a marked decline in IL-1β level by 75% and 64.5% in the heart and brain homogenates, respectively, compared to the diseased group (Fig. 2B).
The effect of Flavo on TNF-α and IL-1β in Cyclo-injected mice. Oral administration of (20 mg/kg) flavocoxid (Flavo) to mice from day 0 to 14 followed by cyclophosphamide (Cyclo) intraperitoneal (100 mg/kg) at day 14. Data were expressed as mean ± SED (n = 10). *, $, #Significantly different compared to normal control, Flavo, and the Cyclo group respectively, using one-way ANOVA test with Tukey–Kramer post hoc test (p < 0.05). (A) Tumor necrosis factor-α (TNF-α) and (B) interleukin (IL) 1β
Influence of Flavo on cTn-I and brain GM-CSF in Cyclo-injected mice
In the Cyclo group (100 mg/kg), there were significant increases in heart troponin I by 84.3% and 84.35% compared with the control and Flavo groups, respectively. However, Flavo protection (20 mg/kg, orally) decreased this level by 26.2% compared with the Cyclo-injected group (Table 3).
After the administration of Cyclo (100 mg/kg), there were significant increases in GM-CSF by 73.82 and 74.89 compared with the control and Flavo groups, respectively. Administration of Flavo (20 mg/kg, orally) before Cyclo significantly decreased GM-CSF by 32.12 compared with the Cyclo-injected group. Oral Flavo alone caused no significant difference in measured biochemical parameters compared with those of the control group (Table 3).
Influence of Flavo on heart NF-κB and brain APP in Cyclo-injected mice
Compared to that in the control group, Cyclo caused a marked elevation in the activity of NF-κB, and the administration of Flavo caused significant suppression of the activity of this complex protein by 61% compared to the Cyclo group (Fig. 3A and B).
The effect of Flavo on heart NF-κB and brain APP in Cyclo-injected mice. Oral administration of (20 mg/kg) flavocoxid (Flavo) to mice from day 0 to 14 followed by cyclophosphamide (Cyclo) intraperitoneal (100 mg/kg) on day 14. Data were expressed as mean ± SED (n = 10). *, $, #Significantly different when compared to normal control, Flavo, and Cyclo group respectively, using one-way ANOVA test with Tukey–Kramer post hoc test (p < 0.05). Western blots bands of (A) Heart NF-κB and (C) Brain APP. Densitometric analyses of relative band intensities. (B) Nuclear factor kappa B (NF-κB) and (D) amyloid precursor protein (APP)
A marked increase in APP level was observed in the Cyclo-administrated group compared with that in the control group. By contrast, there was a clear decrease in brain APP level when administering Flavo compared to Cyclo by 71% (Fig. 3C and D).
Influence of Flavo on cardiac and brain histopathological changes in the Cyclo-injected group
As shown in Fig. 4 (4.1), H&E stained cardiac sections revealed a normal arrangement of cardiomyocytes in the normal control group (A&B) and the group received Flavo alone (C and D). Figure 4 (4.2) shows sections isolated from the Cyclo group with edema widely separated cardiomyocytes (black arrows) (A and B) with focal leukocytic cells infiltration (yellow arrow) in the intoxicated group (C & D). Meanwhile, focal few leukocytic cells infiltration (yellow arrow) was seen in a group protected with Flavo (E and F).
Effect of Flavo on heart and brain histopathological changes in Cyclo-injected mice. 4.1 Microscopic pictures of H&E stained cardiac sections showing a normal arrangement of cardiomyocytes in the control group (A and B) and the group received Flavo (C and D). (A and C) X:100 bar 100; (B and D) X:400 bar 50. 4.2 Microscopic pictures of H&E stained cardiac sections showing edema widely separate cardiomyocytes (black arrows) (A and B) with focal leukocytic cells infiltration (yellow arrow) in the intoxicated group (C and D). Meanwhile, few focal leukocytic cell infiltration (yellow arrow) are seen in the group received Flavo + Cyclo (E and F). (A, C, E) X:100 bar 100; (B, D, F) X:400 bar 50. 4.3 Microscopic pictures of H&E stained cerebral sections showing normal neurons (N) and glial cells (G) in the control group (A) and the group received Flavo (B). The congested blood vessel (red arrows), shrinkage, and degeneration in neurons (black arrows) besides marked glial cell proliferation (yellow arrow) (C and D) is seen in the intoxicated group. Milder neuronal changes and mild glial cell proliferation (yellow arrow) (E and F) are observed in the group that received Flavo + Cyclo. X:400 bar 50. 4.4 Microscopic pictures of H&E stained hippocampal sections showing normal pyramidal neurons in the CA3 region in the control group (A) and the group that received Flavo (B). Marked shrinkage and degeneration in neurons (black arrows) (C) are seen in the intoxicated group. Milder neuronal changes (black arrows) (D) are observed in the group that received Flavo + Cyclo. X:400 bar 50. 4.5 Microscopic pictures of H&E stained cerebellar sections showing normal granular (G), molecular (M), and purkinje (P) cell layers in the control group (A) and the group that received Flavo (B). Marked loss of purkinje neurons (black arrows) (C) is seen in the intoxicated group. Milder changes in purkinje neurons (black arrows) (D) are observed in the group that received Flavo + Cyclo. X:400 bar 50
However, H&E stained cerebral sections showed normal neurons (N) and glial cells (G) in the control group (A) and the group received Flavo alone (B). Congested blood vessels (red arrows), shrinkage, and degeneration in neurons (black arrows), besides the marked glial cells proliferation (yellow arrow) (C & D), were seen in the diseased group. Milder neuronal changes and mild glial cells proliferation (yellow arrow) (E and F) were observed in the group received Flavo plus Cyclo (Fig. 4 (4.3)).
Hippocampal sections revealing normal pyramidal neurons in the CA3 region in the control group (A) and the Flavo (B) groups. Marked shrinkage and degeneration in neurons (black arrows) (C) were seen in the intoxicated group. Milder neuronal changes (black arrows) (D) were observed in the group received Flavo with Cyclo (Fig. 4 (4.4)).
The cerebellar section stained with H&E represented normal granular (G), molecular (M), and purkinje (P) cell layers in the control (A) and Flavo groups (B). Marked loss of purkinje neurons (black arrows) (C) was seen in the diseased group. Milder changes in purkinje neurons (black arrows) (D) were observed in the group received Flavo + Cyclo (Fig. 4 (4.5)).
Influence of Flavo on immunohistochemical staining of cardiac and brain caspase-3
In Fig. 5 (5.1), immunostained cardiac sections against caspase-3 showing negative staining in control (A) and Flavo alone groups (B). The marked positive brown expression appeared in cardiac muscles in the Cyclo group (C) (black arrows) compared with the normal heart group (E). Caspase-3 positive expression markedly decreased in cardiac muscles in the group protected with Flavo compared to the Cyclo group (D and E).
Effect of Flavo on heart and brain caspase-3 in Cyclo-injected mice. 5.1 Microscopic pictures of immunostained cardiac sections against caspase-3 showing negative staining in the control group (A) and group received Flavo alone (B). The marked positive brown expression appears in cardiac muscles in the Cyclo group (C) (black arrows). Caspase-3 positive expression markedly reduced in cardiac muscles in the group protected with Flavo compared to the Cyclo group (D). IHC counterstained with Mayer’s hematoxylin. X:400 bar 50. Scatter-dot plot representing the score of positive staining of cardiac caspase-3 (E). Data are expressed as mean ± SEM (n = 8). *, $, #Significantly different from control, Flavo, and Cyclo groups, respectively. Statistical analysis was conducted using Kruskal–Wallis followed by Dunn’s multiple comparison test. 5.2 Microscopic pictures of immunostained cerebral cortical sections against caspase-3 showing negative staining in the control group (A) and Flavo group (B).The marked positive brown neuronal expression appears in the Cyclo group (C) (black arrows). The neuronal expression against caspase-3 reduced in the protected group with Flavo compared to the Cyclo group (D). IHC counterstained with Mayer’s hematoxylin. X:400 bar 50. Scatter-dot plot demonstrating the score of positive staining of the brain caspase-3 (E). Data are expressed as mean ± SEM (n = 8). *, $, #Significantly different from control, Flavo, and the Cyclo groups, respectively. Statistical analysis was conducted using Kruskal–Wallis followed by Dunn’s multiple comparison test
Cerebral cortical sections immunostained against caspase-3 in Fig. 5 (5.2) revealing negative staining in the control group (A) and Flavo group (B). Significant positive brown neuronal expression appears in the Cyclo group (C and E) (black arrows) when compared to the control group. The neuronal expression against caspase-3 decreased in the group protected with Flavo compared to the Cyclo group (D and E).
Discussion
Cyclo is a major drug for cancer chemotherapy frequently used to treat breast cancer, but many side effects of Cyclo have been reported, such as cardio and neuroinflammation (Kurauchi et al. 2017; Flanigan et al. 2018). Thus, the natural antioxidant Flavo was selected to repair this inflammation due to its antioxidant and anti-inflammatory characteristics and scavenging free radicals with fewer side effects (El-sheakh et al. 2015). Flavo in a 1-g/kg/day dose revealed no-observed-adverse-effect level. In experimental studies, acute and subchronic toxicity indicated that Flavo has an optimal preclinical safety profile. This promising safety profile encourages the use of Flavo in humans. Clinical trials and a postmarketing study reported that Flavo has a significant efficacy in osteoarthritis and good overall and gastrointestinal tolerability, providing a potential therapeutic approach for acute and chronic inflammatory conditions (Abdelaziz et al. 2018). These characteristics were provided by its components, that is, bioflavonoids such as baicalin and catechin (Abdelaziz et al. 2018).
In this study, Flavo at a dose of 20 mg/kg for 14 days significantly improved the heart and brain weight indices; all estimated biochemical markers, such as LDH, CK-MB, NO, MDA, GSH, SOD, TNF-α, IL-1β, cTn-I, NF-κB, GM-CSF, and APP, and immunohistochemical examination of caspase-3 and histological examination of both heart and brain tissues.
Cyclo is considered one of the most common chemotherapeutic agents that induce vascular permeability and edema by changing vascular endothelial cell structure and permeability (Sagawa et al. 2020), and this was evaluated by this study by measuring both cardiac and brain weight indexes.
Compared to the normal group, there was a significant increase in the heart and brain weight index of the diseased group after the Cyclo injection (100 mg/kg, i.p.), whereas there was a significant decrease of these indices in the group treated with Flavo (20 mg/kg, orally) for 14 days. This in line with a previous study in which Flavo reduced prostate weight and inhibited prostate enlargement in a rat model with the same dose because Flavo can inhibit both the COX and 5-LOX enzymes (Altavilla et al. 2012).
Oxidative stress is a phenomenon caused by an imbalance between the production and accumulation of oxygen reactive species (ROS) in cells and tissues and the ability of a biological system to detoxify these reactive products (Pizzino et al. 2017). These free radicals initiate chain reactions producing peroxidative breakdown of polyunsaturated fatty acids in the membrane bilayer. The interaction among oxygen-free radicals with polyunsaturated fatty acids has been known as lipid peroxidation and can be measured by malondialdehyde formation (PB et al. 1984). The accumulation of MDA depletes physiological antioxidants SOD and GSH. This study emphasizes the inflammatory response of Cyclo by raising MDA level compared with the control and Flavo groups. In Motawi et al., Cyclo was injected by dose of 200 mg/kg in rat models, which increased MDA in myocardium testicles and urinary bladder. Similarly, when it was injected at a dose 75 mg/kg i.p in rat, the brain exhibited a high level of MDA (Oboh and Ogunruku 2010). Interestingly, the protected group exhibited lower level of MDA and a higher level of SOD and GSH. The study of Abdelaziz et al. (2018) showed a marked decline in MDA level and relative increase in GSH and SOD when Flavo (20 mg/kg) for 16 days was used in an ovalbumin-induced mouse asthma model.
LDH is an important enzyme involved in energy production, and it is a good biomarker indicating cell damage (El-Agamy et al. 2017). Additionally, CK-MB is an enzyme found mostly in the heart and presents with significantly high amounts during cardiac damage (Cengiz et al. 2020).
In this study, there was a significant increase in LDH and CK-MB activities after only administration of Cyclo compared with the control group, which reflex the severe cardiotoxicity of Cyclo that is ensured by results of previous studies (El-Agamy et al. 2017; Gunes et al. 2017). The reason is that Cyclo is cardiotoxic by causing endothelial dysfunction and cardiomyocytes damage. Moreover, Cyclo causes lipid peroxidation, which disturbs endothelial cell permeability, resulting in elevated serum LDH and CK-MB levels (Cengiz et al. 2020). However, by the protective administration of Flavo with Cyclo, serum levels of LDH and CK-MB significantly diminished compared with the Cyclo-treated group.
Another biomarker contributing to Cyclo-induced cardiotoxicity is nitric oxide (NO). Although NO has an anti-inflammatory effect physiologically, NO plays an essential role in the pathogenesis of inflammation and oxidative stress in abnormal conditions (Papi et al. 2019). The current study found significantly high level of NO after a single dose of Cyclo compared with control, which aligns with previous studies (Iqubal et al. 2019b; Mansour et al. 2015). The explanation can be that acrolein, a metabolite of Cyclo, causes an increase in endothelial nitric oxide synthase (eNOS) monomers relative to eNOS dimers leading to eNOS uncoupling and, consequently, increase in nitric oxide and nitrative stress (Ismahil et al. 2011). By the protective administration of the Flavo with Cyclo, serum NO levels significantly decreased compared with the Cyclo-treated group. This result is in parallel with a previous study showing the ability of Flavo (20 mg/kg, i.p.) to improve NO levels in ovalbumin-induced asthma in mice (Abdelaziz et al. 2018).
Tumor necrosis factor-α (TNF-α) is an inflammatory cytokine that resulted from macrophages/monocytes during acute inflammation and is responsible for a diverse range of cell signaling events (Hartupee and Mann 2013). While IL-1β is a proinflammatory cytokine that has been involved in inflammation, pain, and autoimmune conditions (Mohan 2016). This work showed a significant increase in both heart and brain TNF-α and IL-1β by Cyclo (100 mg/kg, i.p.) compared with the control and Flavo groups, and this is in parallel with the study of Iqubal et al. (2020), which investigated that the Cyclo-induced neurotoxicity by the dose of 200 mg/kg in mice model through increasing the TNF-α and IL-1β significantly. Flavo decremented both levels in brain and heart homogenates in protected group compared with the Cyclo group, and this was in agreement with a study in which Flavo with dose (50, 100, 200 mg/kg; p.o.) in rat ischemic stroke model showed a significant decrease in TNF-α and IL-1β, and has a neuroprotective effect (Singh and Chopra 2014).
Cardiac Troponin I (cTn-I) represents a highly sensitive marker for myocardial cell death. This study shows the significant increase in cardiac troponin I with the Cyclo in heart homogenates compared to the control and Flavo groups. This is consistent with the previous study that showed high level of cTn-I following administration of Cyclo with a dose of 100 mg/kg in mice model (Iqubal, et al. 2019b). While Flavo with dose (20 mg/kg) alleviated cTn-I compared with the Cyclo group.
Nuclear factor kappa B (NF-κB) is considered a key transcription factor involved in many inflammatory disorders, such as cardiac disorder. It targets the proinflammatory genes and drives the expression of these genes leading to an elevation in production of cytokines and other proinflammatory proteins from cardiac tissue (Song et al. 2016). In the present study, there was a significant increase in heart NF-κB level after injection of Cyclo (100 mg/kg, i.p), and this coordinated with (Song et al. 2016) the work. Cyclo is considered a cardiotoxic agent that causes myocyte damage and extravasation of toxic metabolites (Dřímal et al. 2006). Otherwise, there was a marked decrease in heart NF-κB level after Flavo (20 mg/kg). This was illustrated by a previous study that referred to Flavo as an oral NF-κB inhibitor through its additional antioxidant effect (Vita et al. 2021). These previous results revealed for the first time the cardioprotective effect of Flavo by significantly improving previous cardiac toxicity biomarkers.
Granulocyte macrophage colony stimulating factor (GM-CSF) is now best viewed as a major regulator governing the functions of granulocyte and macrophage lineage populations at all stages of maturation. Hamilton and Hamilton (2002) study illustrated the crucial role of GM-CSF in inflammatory and autoimmune disease. The present work showed a significant increase in GM-CSF by Cyclo with dose (100 mg/kg) in brain homogenates compared to control and Flavo groups. Flavo with dose (20 mg/kg) alleviated brain GM-CSF compared to the Cyclo group.
APP services as a cell surface receptor in neurons at both presynaptic and postsynaptic sites, which are responsible for many physiological functions such as motility, cell growth, neurite outgrowth, neuronal adhesion, and cell survival through its large insoluble ectodomains; (sAPPα) and (sAPPβ) after breaking by α, β, and γ-secretase (Wolfe et al. 1999; Young-Pearse et al. 2007). APP was defined as a potentially effective biomarker indicating neuropathological alteration in the brain, and this was confirmed by the study of Araki et al. (2017). After injection of Cyclo (100 mg/kg, i.p), there was a significant increase in APP production level in comparison with the normal group, and this agreed with that of Butterfield et al. (2001) study who reported that the accumulation of free radical-oxidative stress and activation of neural lipid peroxidation is associated with the accumulation of APP, which worsens the neurodegenerative action of the Cyclo (Oboh and Ogunruku 2010; Flanigan et al. 2018). There was a significant decrease in APP level on administering Flavo (20 mg/kg, orally) for 2 weeks because Flavo can scavenge free radicals caused by Cyclo and blocks 5-LOX pathway, in addition to decreasing phosphorylation/expression level of the amyloid protein and this integrated with work of (Bitto et al. 2017).
DNA damage and membrane peroxidation resulted from mitochondrial free radicals that promotes outer membrane permeabilization and facilitates the translocation of Bax and cytochrome C from the mitochondria to the cytosol with subsequent caspase activation. Caspase-3 promotes conformational changes and that initiates and executes apoptosis (Hochhauser et al. 2015). In the research of El-Agamy et al. (2017), the cardiac caspase-3 expression increased significantly after single Cyclo administration (200 mg/kg, i.p.) compared with the control. This is parallel with our work in which Cyclo elevated both cardiac and brain caspase-3 expressions, which was indicated by high immunoreactivity area. It was illustrated that Cyclo-induced cardiomyopathy via DNA intercalation, p53 protein activation, and ROS generation, which initiates the apoptotic pathway and then massive apoptosis of cardiomyocytes (El-Agamy et al. 2017).
Flavo administration decreased histological damage and caspase-3 in both heart and brain tissue in this study. This result is consistent with the work of Bitto et al. (2012) in which Flavo in a dose of 20 mg/kg improved pathological changes associated with sepsis in mice and apoptosis through inhibition of mitogen-activated protein kinases pathway, preserved β arrestin-2 expression, TNF-α, NF-κB, and increased IL-10 as well as lipoxin A4.
Conclusion
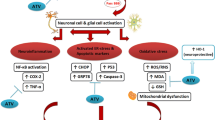
Flavo administration attenuated cardiac/brain injury and ameliorated oxidative stress, inflammation, and apoptosis in the heart and brain, potentially by disrupting multiple signaling pathways mediated by GM-CSF/NF-κB, inflammatory cytokines (TNF-α and IL-1β), and apoptotic markers (caspase-3) (Fig. 6).
Data availability
The data that support the findings of this study are available on request from the corresponding author (Rania R. Abdelaziz). The data are not publicly available due to privacy.
Abbreviations
- 5-LOX :
-
Lipoxygenase
- APP:
-
Amyloid precursor protein
- CK-MB:
-
Creatine kinase
- COX:
-
Cyclooxygenase
- Cyclo:
-
Cyclophosphamide
- cTn-I:
-
Cardiac troponin I
- Flavo:
-
Flavocoxid
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- GSH:
-
Reduced glutathione
- H&E:
-
Hematoxylin and eosin
- IHC:
-
Immunohistochemistry
- IL-1β:
-
Interleukin-1β
- i.p.:
-
Intraperitoneal
- LDH:
-
Lactate dehydrogenase
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNF-α :
-
Tumor necrosis factor-α
References
Abdelaziz RR, Elmahdy M, Suddek GM (2018) Flavocoxid attenuates airway inflammation in ovalbumin-induced mouse asthma model. Chem Biol Interact 292:15–23. https://doi.org/10.1016/j.cbi.2018.07.001
Aladaileh SH, Abukhalil MH, Saghir SAM, Hanieh H, Alfwuaires MA, Almaiman AA, Bin-Jumah M, Mahmoud AM (2019) Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 9. https://doi.org/10.3390/biom9080346
Altavilla D, Squadrito F, Bitto A, Polito F, Burnett BP, Di Stefano V, Minutoli L (2009) Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophages. Br J Pharmacol 157:1410–1418. https://doi.org/10.1111/j.1476-5381.2009.00322.x
Altavilla D, Minutoli L, Polito F, Irrera N, Arena S, Magno C, Rinaldi M, Burnett BP, Squadrito F, Bitto A (2012) Effects of flavocoxid, a dual inhibitor of COX and 5-lipoxygenase enzymes, on benign prostatic hyperplasia. Br J Pharmacol 167:95–108. https://doi.org/10.1111/j.1476-5381.2012.01969.x
Amin FM, Abdelaziz RR, Hamed MF, Nader MA, Shehatou GSG (2020) Dimethyl fumarate ameliorates diabetes-associated vascular complications through ROS-TXNIP-NLRP3 inflammasome pathway. Life Sci 256:117887. https://doi.org/10.1016/j.lfs.2020.117887
Araki W, Hattori K, Kanemaru K, Yokoi Y, Omachi Y, Takano H, Sakata M, Yoshida S, Tsukamoto T, Murata M, Saito Y, Kunugi H, Goto YI, Nagaoka U, Nagao M, Komori T, Arima K, Ishii K, Murayama S, Matsuda H, Tachimori H, Araki YM, Mizusawa H (2017) Re-evaluation of soluble APP-α and APP-β in cerebrospinal fluid as potential biomarkers for early diagnosis of dementia disorders. Biomarker Res 5:1–9. https://doi.org/10.1186/s40364-017-0108-5
Avci H, Epikmen ET, Ipek E, Tunca R, Birincioglu SS, Akşit H, Sekkin S, Akkoç AN, Boyacioglu M (2017) Protective effects of silymarin and curcumin on cyclophosphamide-induced cardiotoxicity. Exp Toxicol Pathol 69:317–327. https://doi.org/10.1016/j.etp.2017.02.002
Bitto A, Minutoli L, David A, Irrera N, Rinaldi M, Venuti FS, Squadrito F, Altavilla D (2012) Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit Care 16:1–12. https://doi.org/10.1186/1364-8535-16-R32
Bitto A, Giuliani D, Pallio G, Irrera N, Vandini E, Canalini F, Zaffe D, Ottani A, Minutoli L, Rinaldi M, Guarini S, Squadrito F, Altavilla D (2017) Effects of COX1-2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm Res 66:389–398. https://doi.org/10.1007/s00011-017-1022-x
Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT (1991) Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol 9(7):1215–1223. https://doi.org/10.1200/JCO.1991.9.7.1215
Burnett BP, Bitto A, Altavilla D, Squadrito F, Levy RM, Pillai L (2011) Flavocoxid inhibits phospholipase A2, peroxidase moieties of the cyclooxygenases (COX), and 5-lipoxygenase, modifies COX-2 gene expression, and acts as an antioxidant. Mediators Inflamm. 2011. https://doi.org/10.1155/2011/385780
Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid β-peptide. Trends Mol Med 7:548–554. https://doi.org/10.1016/S1471-4914(01)02173-6
Cengiz M, Kutlu HM, Peker Cengiz B, Ayhancı A (2020) Escin attenuates oxidative damage, apoptosis and lipid peroxidation in a model of cyclophosphamide-induced liver damage. Drug Chem Toxicol: 1–8. https://doi.org/10.1080/01480545.2020.1810262
Chen XZ, Cao ZY, Zhang YQ, Li JN, Liao LM, Du J (2017) Fuzheng qingjie granules potentiate the anticancer effect of cyclophosphamide by regulating cellular immune function and inducing apoptosis in hepatoma 22 tumor-bearing mice. Oncol Lett 13:3261–3268. https://doi.org/10.3892/ol.2017.5849
Chen L, Xiong X, Hou X, Wei H, Zhai J, Xia T, Gong X, Gao S, Feng G, Tao X, Zhang F, Chen W (2019) Wuzhi capsule regulates chloroacetaldehyde pharmacokinetics behaviour and alleviates high-dose cyclophosphamide-induced nephrotoxicity and neurotoxicity in rats. Basic Clin Pharmacol Toxicol 125:142–151. https://doi.org/10.1111/bcpt.13211
Dřímal J, Zúrová-Nedelčevová J, Knezl V, Sotníková R, Navarová J (2006) Cardiovascular toxicity of the first line cancer chemotherapeutic agents: doxorubicin cyclophosphamide, streptozotocin and bevacizumab. Neuroendocrinol Lett 27:176–179
El-Agamy DS, Elkablawy MA, Abo-Haded HM (2017) Modulation of cyclophosphamide-induced cardiotoxicity by methyl palmitate. Cancer Chemother Pharmacol 79:399–409. https://doi.org/10.1007/s00280-016-3233-1
El-sheakh AR, Ghoneim HA, Suddek GM (2015) Antioxidant and anti-inflammatory effects of flavocoxid in high-cholesterol-fed rabbits 1333–1344. https://doi.org/10.1007/s00210-015-1168-4
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Elmariah H, Kasamon YL, Zahurak M, Macfarlane KW, Tucker N, Rosner GL, Bolaños-Meade J, Fuchs EJ, Wagner-Johnston N, Swinnen LJ, Huff CA, Matsui WH, Gladstone DE, McCurdy SR, Borrello I, Gocke CB, Shanbhag S, Cooke KR, Ali SA, Brodsky RA, DeZern AE, Luznik L, Jones RJ, Ambinder RF (2018) Haploidentical bone marrow transplantation with post-transplant cyclophosphamide using non–first-degree related donors. Biol Blood Marrow Transplant 24:1099–1102. https://doi.org/10.1016/j.bbmt.2018.02.005
Flanigan TJ, Anderson JE, Elayan I, Allen AR, Ferguson SA (2018) Effects of cyclophosphamide and/or doxorubicin in a murine model of postchemotherapy cognitive impairment. Toxicol Sci 162:462–474. https://doi.org/10.1093/toxsci/kfx267
Goldberg MA, Antin JH, Guinan EC, Rappeport JM (1986) Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor
Gown AM, Willingham MC (2002) Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem
Gunes S, Sahinturk V, Karasati P, Sahin IK, Ayhanci A (2017) Cardioprotective effect of selenium against cyclophosphamide-induced cardiotoxicity in rats. Biol Trace Elem Res 177:107–114. https://doi.org/10.1007/s12011-016-0858-1
Hamilton JA, Hamilton JA (2002) <Hamilton Trends Immunol 2002.pdf> 23: 403–408
Hartupee J, Mann DL (2013) Positioning of inflammatory biomarkers in the heart failure landscape. J Cardiovasc Transl Res 6:485–492. https://doi.org/10.1007/S12265-013-9467-Y
Hochhauser E, Lahat E, Sultan M, Pappo O, Waldman M, Sarne Y, Shainberg A, Gutman M, Safran M, Ari ZB (2015) Ultra low dose delta 9-tetrahydrocannabinol protects mouse liver from ischemia reperfusion injury. Cell Physiol Biochem 36:1971–1981. https://doi.org/10.1159/000430165
Iqubal A, Iqubal MK, Sharma S, Ansari MA, Najmi AK, Ali SM, Ali J, Haque SE (2019a) Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci. https://doi.org/10.1016/j.lfs.2018.12.018
Iqubal A, Sharma S, Ansari MA, Najmi AK, Syed MA, Ali J, Alam MM, Ahmad S, Haque SE (2019b) Nerolidol attenuates cyclophosphamide-induced cardiac inflammation, apoptosis and fibrosis in Swiss Albino mice. Eur J Pharmacol 863. https://doi.org/10.1016/j.ejphar.2019.172666
Iqubal A, Syed MA, Najmi AK, Azam F, Barreto GE, Iqubal MK, Ali J, Haque SE (2020) Nano-engineered nerolidol loaded lipid carrier delivery system attenuates cyclophosphamide neurotoxicity — probable role of NLRP3 inflammasome and caspase-1. Exp Neurol 334:113464. https://doi.org/10.1016/J.EXPNEUROL.2020.113464
Ismahil MA, Hamid T, Haberzettl P, Gu Y, Chandrasekar B, Srivastava S, Bhatnagar A, Prabhu SD (2011) Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 301:2050–2060. https://doi.org/10.1152/ajpheart.00120.2011
Kurauchi K, Nishikawa T, Miyahara E, Okamoto Y, Kawano Y (2017) Role of metabolites of cyclophosphamide in cardiotoxicity. BMC Res Notes. https://doi.org/10.1186/s13104-017-2726-2
Mansour HH, El kiki SM, Hasan HF (2015) Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol 40:417–422. https://doi.org/10.1016/j.etap.2015.07.013
Maresh MM, Abdelaziz RR, Ibrahim TM (2020) Febuxostat mitigates concanavalin A-induced acute liver injury via modulation of MCP-1, IL-1β, TNF-α, neutrophil infiltration, and apoptosis in mice. Life Sci 260:118307. https://doi.org/10.1016/j.lfs.2020.118307
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Messina S, Bitto A, Aguennouz M, Mazzeo A, Migliorato A, Polito F, Irrera N, Altavilla D, Vita GL, Russo M, Naro A, De Pasquale MG, Rizzuto E, Musarò A, Squadrito F, Vita G (2009) Flavocoxid counteracts muscle necrosis and improves functional properties in mdx mice: a comparison study with methylprednisolone. Exp Neurol 220:349–358. https://doi.org/10.1016/j.expneurol.2009.09.015
Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S (2015) Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci 143:242–255. https://doi.org/10.1093/toxsci/kfu233
Mohan S (2016) Interleukin-1beta: a common thread between inflammation, pain and opioid tolerance. Neuroimmunol Neuroinflammation 3:201–203
Oboh G, Ogunruku OO (2010) Cyclophosphamide-induced oxidative stress in brain: protective effect of hot short pepper (Capsicum frutescens L. var. abbreviatum). Exp Toxicol Pathol 62:227–233. https://doi.org/10.1016/J.ETP.2009.03.011
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Papi S, Ahmadizar F, Hasanvand A (2019) The role of nitric oxide in inflammation and oxidative stress. Immunopathol Persa 5:e08–e08. https://doi.org/10.15171/ipp.2019.08
Pb C, Rw G, Be S (1984) Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res 55:135–154. https://doi.org/10.1161/01.RES.55.2.135
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017. https://doi.org/10.1155/2017/8416763
Polito F, Bitto A, Irrera N, Squadrito F, Fazzari C, Minutoli L, Altavilla D (2010) Flavocoxid, a dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, reduces pancreatic damage in an experimental model of acute pancreatitis. Br J Pharmacol 161:1002–1011. https://doi.org/10.1111/j.1476-5381.2010.00933.x
Rzeski W, Pruskil S, Macke A, Felderhoff-Mueser U, Reiher AK, Hoerster F, Jansma C, Jarosz B, Stefovska V, Bittigau P, Ikonomidou C (2004) Anticancers agents are potent neurotoxins in vitro and in vivo. Ann Neurol 56:351–360. https://doi.org/10.1002/ana.20185
Sagawa N, Oshima Y, Hiratsuka T, Kono Y, Etoh T, Inomata M (2020) Role of increased vascular permeability in chemotherapy-induced alopecia: In vivo imaging of the hair follicular microenvironment in mice. https://doi.org/10.1111/cas.14396
Seo EJ, Klauck SM, Efferth T, Panossian A (2019) Adaptogens in chemobrain (Part II): effect of plant extracts on chemotherapy-induced cytotoxicity in neuroglia cells. Phytomedicine 58. https://doi.org/10.1016/j.phymed.2018.11.004
Singh DP, Chopra K (2014) Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, exhibits neuroprotection in rat model of ischaemic stroke. Pharmacol Biochem Behav 120:33–42. https://doi.org/10.1016/J.PBB.2014.02.006
Singh S, Kumar A (2019) Protective effect of edaravone on cyclophosphamide induced oxidative stress and neurotoxicity in Rats. Curr Drug Saf 14:209–216. https://doi.org/10.2174/1574886314666190506100717
Song Y, Zhang C, Wang C, Zhao L, Wang Z, Dai Z, Lin S, Kang H, Ma X (2016) Ferulic acid against cyclophosphamide-induced heart toxicity in mice by inhibiting NF-B pathway. Evid Based Complement Alternat Med 2016. https://doi.org/10.1155/2016/1261270
Vita GL, Sframeli M, Licata N, Bitto A, Romeo S, Frisone F, Ciranni A, Pallio G, Mannino F, Aguennouz M, Rodolico C, Squadrito F, Toscano A, Messina S, Vita G (2021) A phase 1/2 study of flavocoxid, an oral NF-κb inhibitor, in duchenne muscular dystrophy. Brain Sci 11:1–12. https://doi.org/10.3390/brainsci11010115
Wolfe MS, De Los Angeles J, Miller DD, Xia W, Selkoe DJ (1999) Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer’s disease. Biochemistry 38:11223–11230. https://doi.org/10.1021/bi991080q
Würzburg U, Hennrich N, Orth H-D, Lang H, Prellwitz W, Neumeier D, Knedel M, Rick W (1977) Quantitative determination of 1 creatine kinase isoenzyme catalytic concentrations in serum using immunological methods. J Clin Chem Clin Biochem 15:131–137
Young-Pearse TL, Bai J, Chang R, Zheng JB, Loturco JJ, Selkoe DJ (2007) A critical function for β-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 27:14459–14469. https://doi.org/10.1523/JNEUROSCI.4701-07.2007
Zhai J, Zhang F, Gao S, Chen L, Feng G, Yin J, Chen W (2018) Schisandra chinensis extract decreases chloroacetaldehyde production in rats and attenuates cyclophosphamide toxicity in liver, kidney and brain. J Ethnopharmacol 210:223–231. https://doi.org/10.1016/j.jep.2017.08.020
Acknowledgements
Great thanks to Walaa F. Awadin, associated professor of Pathology, Faculty of Veterinary Medicine, Mansoura University, for his help to complete the histopathological and IHC parts of this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Rania R. Abdelaziz: supervision, conceptualization, conceived, designed research, methodology, software, writing- reviewing, editing, and approved the manuscript. Fatma F. Elsayed and Waad M. Elshenawy: conducted experiments, data curation, writing- original draft preparation, software, and validation. Eman M. Khalifa and Mohamed R. Rizq: conducted experiments, software, validation, visualization, investigation.
Corresponding author
Ethics declarations
Ethical approval
Experiment was performed following the rules and ethical standards for treatment and use of laboratory animals established by Research Ethical Committee, Faculty of Pharmacy, and Mansoura University.
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsayed, F.F., Elshenawy, W.M., Khalifa, E.M. et al. Ameliorative effect of flavocoxid on cyclophosphamide-induced cardio and neurotoxicity via targeting the GM-CSF/NF-κB signaling pathway. Environ Sci Pollut Res 29, 69635–69651 (2022). https://doi.org/10.1007/s11356-022-20441-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20441-5