Abstract
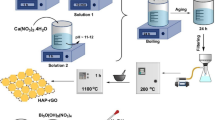
A novel composite (nZVI/Pd-AC) was prepared by loading nanoscale zero-valent iron (nZVI) and Pd on activated carbon (AC) electrode under electrodeposition with ultrasound, which was used to reductive degradation of methylene blue (MB). The loading contents of Fe and Pd in composite materials were 15.84% and 2.06%, respectively. XPS results further confirmed that the as-prepared material contained Fe0 and Pd0. Without external conditions, MB could be degraded in the presence of nZVI/Pd-AC and reached equilibrium within 180 min. To investigate the reusability, the re-electrodeposition strategy was effective to refresh the active sites of nZVI/Pd-AC, and the removal efficiency only reduced by 4.51% in five circles indicating the good reusability of nZVI/Pd-AC composites. GC–MS was used to identify possible degradation pathways of MB; the results showed that the degradation products were mainly N, N-dimethylaniline and 2-amino-5-dimethylamino-benzenesulfonic acid. And the S–C, C–N bonds are the sites easier to be attacked.
Graphical abstract








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this manuscript.
References
Berge ND, Ramsburg CA (2009) Oil-in-water emulsions for encapsulated delivery of reactive iron particles. Environ Sci Technol 43:5060–5066
Bhaumik M, Maity A, Gupta VK (2017) Synthesis and characterization of Fe0/TiO2 nano-composites for ultrasound assisted enhanced catalytic degradation of reactive black 5 in aqueous solutions. J Colloid Interface Sci 506:403–414
Burkinshaw SM, Salihu G (2019a) The role of auxiliaries in the immersion dyeing of textile fibres part 2: analysis of conventional models that describe the manner by which inorganic electrolytes promote direct dye uptake on cellulosic fibres. Dyes Pigments 161:531–545
Burkinshaw SM, Salihu G (2019b) The role of auxiliaries in the immersion dyeing of textile fibres: Part 6 analysis of conventional models that describe the manner by which inorganic electrolytes promote reactive dye uptake on cellulosic fibres. Dyes Pigments 161:595–604
Chen S-S, Hsu H-D, Li C-W (2005) A new method to produce nanoscale iron for nitrate removal. J Nanopart Res 6:639–647
Costa JM, Almeida Neto AF (2020) Ultrasound-assisted electrodeposition and synthesis of alloys and composite materials: a review. Ultrason Sonochem 68:105193
Domenichini P, Condó AM, Haberkorn N (2016) Structural characterization of FePd nanowires grown by electrodeposition using an acid electrolyte. Mater Chem Phys 177:164–170
Fan J, Guo Y, Wang J, Fan M (2009) Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles. J Hazard Mater 166:904–910
Frost RL, Xi Y, He H (2010) Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J Colloid Interface Sci 341:153–161
Fu Y, Peng L, Zeng Q, Yang Y, Song H, Shao J, Liu S, Gu J (2015) High efficient removal of tetracycline from solution by degradation and flocculation with nanoscale zerovalent iron. Chem Eng J 270:631–640
He F, Zhao D (2007) Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41:6216–6221
Huang FM, Chen L, Wang HL, Yan ZC (2010) Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem Eng J 162:250–256
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods: a review. J Environ Chem Eng 6:4676–4697
Lai B, Zhang Y, Chen Z, Yang P, Zhou Y, Wang J (2014) Removal of p-nitrophenol (PNP) in aqueous solution by the micron-scale iron–copper (Fe/Cu) bimetallic particles. Appl Catal B Environ 144:816–830
Li X-q, Zhang W-x (2006) Iron nanoparticles: the core−shell structure and unique properties for Ni(II) sequestration. Langmuir 22:4638–4642
Li S, Wang W, Liang F, Zhang WX (2017) Heavy metal removal using nanoscale zero-valent iron (nZVI): theory and application. J Hazard Mater 322:163–171
Li J, Wang H, Wang L, Ma C, Luan C, Zhao B, Zhang Z, Zhang H, Cheng X, Liu J (2018) The preparation of Pd/foam-Ni electrode and its electrocatalytic hydrodechlorination for monochlorophenol isomers. Catalysts 8:378
Lin J, Weng X, Jin X, Megharaj M, Naidu R, Chen Z (2015) Reactivity of iron-based nanoparticles by green synthesis under various atmospheres and their removal mechanism of methylene blue. RSC Adv 5:70874–70882
Liu J, Liu A, Zhang W-x (2016) The influence of polyelectrolyte modification on nanoscale zero-valent iron (nZVI): aggregation, sedimentation, and reactivity with Ni(II) in water. Chem Eng J 303:268–274
Nairat M, Shahwan T, Eroğlu AE, Fuchs H (2015) Incorporation of iron nanoparticles into clinoptilolite and its application for the removal of cationic and anionic dyes. J Ind Eng Chem 21:1143–1151
Perez-Mayoral E, Calvino-Casilda V, Soriano E (2016) Metal-supported carbon-based materials: opportunities and challenges in the synthesis of valuable products. Catal Sci Technol 6:1265–1291
Qian LB, Zhang WY, Yan JC, Han L, Chen Y, Ouyang D, Chen MF (2017) Nanoscale zero-valent iron supported by biochars produced at different temperatures: synthesis mechanism and effect on Cr(VI) removal. Environ Pollut 223:153–160
Rajeswari A, Christy EJS, Mary GIC, Jayaraj K, Pius A (2019) Cellulose acetate based biopolymeric mixed matrix membranes with various nanoparticles for environmental remediation-a comparative study. J Environ Chem Eng 7:103278
Rodrigues R, Betelu S, Colombano S, Masselot G, Tzedakis T, Ignatiadis I (2017) Reductive dechlorination of hexachlorobutadiene by a Pd/Fe microparticle suspension in dissolved lactic acid polymers: degradation mechanism and kinetics. Ind Eng Chem Res 56:12092–12100
Satapanajaru T, Chompuchan C, Suntornchot P, Pengthamkeerati P (2011) Enhancing decolorization of Reactive Black 5 and Reactive Red 198 during nano zerovalent iron treatment. Desalination 266:218–230
Shi J, Long C, Li A (2016) Selective reduction of nitrate into nitrogen using Fe–Pd bimetallic nanoparticle supported on chelating resin at near-neutral pH. Chem Eng J 286:408–415
Shu H-Y, Chang M-C, Yu H-H, Chen W-H (2007) Reduction of an azo dye Acid Black 24 solution using synthesized nanoscale zerovalent iron particles. J Colloid Interface Sci 314:89–97
Shu HY, Chang MC, Chen CC, Chen PE (2010) Using resin supported nano zero-valent iron particles for decoloration of Acid Blue 113 azo dye solution. J Hazard Mater 184:499–505
Tang L, Tang J, Zeng G, Yang G, Xie X, Zhou Y, Pang Y, Fang Y, Wang J, Xiong W (2015) Rapid reductive degradation of aqueous p-nitrophenol using nanoscale zero-valent iron particles immobilized on mesoporous silica with enhanced antioxidation effect. Appl Surf Sci 333:220–228
Tian H, Liang Y, Zhu T, Zeng X, Sun Y (2018) Surfactant-enhanced PEG-4000-NZVI for remediating trichloroethylene-contaminated soil. Chemosphere 195:585–593
Tudela I, Zhang Y, Pal M, Kerr I, Mason TJ, Cobley AJ (2015) Ultrasound-assisted electrodeposition of nickel: effect of ultrasonic power on the characteristics of thin coatings. Surf Coat Technol 264:49–59
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manag 93:154–168
Wang T, Su J, Jin X, Chen Z, Megharaj M, Naidu R (2013) Functional clay supported bimetallic nZVI/Pd nanoparticles used for removal of methyl orange from aqueous solution. J Hazard Mater 262:819–825
Wang C, Liu T, Bu F, Liu H (2015a) Perchlorate removal using a minimized dosage of electrodeposited zero-valent iron. J Environ Eng-ASCE 141:04014064
Wang X, Wang P, Ma J, Liu H, Ning P (2015b) Synthesis, characterization, and reactivity of cellulose modified nano zero-valent iron for dye discoloration. Appl Surf Sci 345:57–66
Wang P, Wang X, Yu S, Zou Y, Wang J, Chen Z, Alharbi NS, Alsaedi A, Hayat T, Chen Y, Wang X (2016) Silica coated Fe 3 O 4 magnetic nanospheres for high removal of organic pollutants from wastewater. Chem Eng J 306:280–288
Wu J, Yi Y, Li Y, Fang Z, Tsang EP (2016) Excellently reactive Ni/Fe bimetallic catalyst supported by biochar for the remediation of decabromodiphenyl contaminated soil: reactivity, mechanism, pathways and reducing secondary risks. J Hazard Mater 320:341–349
Wu Y, Yue Q, Gao Y, Ren Z, Gao B (2018) Performance of bimetallic nanoscale zero-valent iron particles for removal of oxytetracycline. J Environ Sci 69:173–182
Xi Y, Megharaj M, Naidu R (2011) Dispersion of zerovalent iron nanoparticles onto bentonites and use of these catalysts for orange II decolourisation. Appl Clay Sci 53:716–722
Xiong Z, Zhao D, Pan G (2007) Rapid and complete destruction of perchlorate in water and ion-exchange brine using stabilized zero-valent iron nanoparticles. Water Res 41:3497–3505
Xue YH, Xiang P, Wang HW, Long YT, Lian HL, Shi WX (2019) Mechanistic insights into selective adsorption and separation of multi-component anionic dyes using magnetic zeolite imidazolate framework-67 composites. J Mol Liq 296:111990
Yan W, Lien H-L, Koel BE, Zhang W-x (2013) Iron nanoparticles for environmental clean-up: recent developments and future outlook. Environ Sci Process Impacts 15:63–77
Yang GCC, Lee H-L (2005) Chemical reduction of nitrate by nanosized iron: kinetics and pathways. Water Res 39:884–894
Yang B, Tian Z, Zhang L, Guo Y, Yan S (2015) Enhanced heterogeneous Fenton degradation of Methylene Blue by nanoscale zero valent iron (nZVI) assembled on magnetic Fe3O4/reduced graphene oxide. J Water Process Eng 5:101–111
Zeng Y, Walker H, Zhu Q (2017) Reduction of nitrate by NaY zeolite supported Fe, Cu/Fe and Mn/Fe nanoparticles. J Hazard Mater 324:605–616
Zhang X, Lin Y-m, Z-l C (2009) 2,4,6-Trinitrotoluene reduction kinetics in aqueous solution using nanoscale zero-valent iron. J Hazard Mater 165:923–927
Zhao R, Wang Y, Li X, Sun B, Wang C (2015) Synthesis of beta-cyclodextrin-based electrospun nanofiber membranes for highly efficient adsorption and separation of Methylene Blue. ACS Appl Mater Interfaces 7:26649–26657
Zhou Y, Li Z, Hao C, Zhang Y, Chai S, Han G, Xu H, Lu J, Dang Y, Sun X, Fu Y (2020) Electrocatalysis enhancement of α, β-PbO2 nanocrystals induced via rare earth Er(III) doping strategy: principle, degradation application and electrocatalytic mechanism. Electrochim Acta 333:135535
Zhu F, Li L, Ma S, Shang Z (2016) Effect factors, kinetics and thermodynamics of remediation in the chromium contaminated soils by nanoscale zero valent Fe/Cu bimetallic particles. Chem Eng J 302:663–669
Zhu F, Ma S, Liu T, Deng X (2018) Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J Clean Prod 174:184–190
Acknowledgements
The author acknowledges the Key Laboratory of the Three Gorges Reservoir Region’s Eco-Environment for providing all the support during the study period. The author also acknowledges Hongxia Liu for his valuable suggestions and helpful advice on various technical issues examined in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Yingtao Long: designed and performed the experiments, analyzed data, and wrote the manuscript. Jianjun Liang: supervised the research and edited the paper. Yinghao Xue: writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research and ethical standards.
Competing interests
The authors disclose any actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations within 3 years of the beginning of the submitted work that could inappropriately influence, or perceived to influence, this work.
Consent for publication
The authors grant the publisher the sole and exclusive license of the full copyright of this contribution.
Additional information
Responsible Editor: Weiming Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• nZVI-Pd/AC is prepared by electrodeposition.
• The degradation of methylene blue dye by nZVI-Pd/AC is satisfactory.
• Remarkable performance and reusability were shown in the degradation of dyes under the influence of various factors.
• Fe0 reduction with enhancement of Pd catalysis plays key roles in the adsorption process.
Rights and permissions
About this article
Cite this article
Long, Y., Liang, J. & Xue, Y. Ultrasound-assisted electrodeposition synthesis of nZVI-Pd/AC toward reductive degradation of methylene blue. Environ Sci Pollut Res 28, 67098–67107 (2021). https://doi.org/10.1007/s11356-021-15316-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15316-0