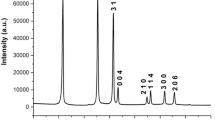
Abstract
The present study has been conducted to evaluate the potential threat of NiO nanoparticles (NiO NPs) on an edible fish Heteropneustes fossilis. Fishes selected for the study were exposed to four concentrations of NiO NPs (12, 24, 36 and 48 mg/l) for the period of 14 days, and various haematological, biochemical and enzymological changes in the exposed fishes were examined. Results revealed that maximum fluctuations were seen in 48-mg/l-exposed fishes when compared with the control in terms of the haematological parameters (RBC count, WBC count, Hb content, Ht% and O2 carrying capacity of blood), enzymatic activities (AST, ALP, ALT and LDH) and biochemical parameters (level of cholesterol, triglycerides, glucose, total protein, albumin, globulin, bilirubin and creatinine). However, 12 mg/l treatment to the fishes showed its least impact on aforesaid parameters. Furthermore, Ni accumulation and changes in cortisol level in the blood were also noticed in all the treated fishes. Structural changes, such as membrane and nuclear disintegration, micronucleus, deformed and vacuolated cells, and enucleation were also observed in RBCs of NiO NP–treated fishes. Conclusively, our study provides useful information and insight for the possible ecotoxicity of NiO NPs on aquatic organisms and emphasizes upon the importance of treatment of effluents containing nanoparticles before their release into the aquatic system.







Similar content being viewed by others
References
Abdel-Khalek AA, Kadry MAM, Badran S, Marie MAS (2015) Comparative toxicity of copper oxide bulk and nano particles in Nile Tilapia; Oreochromis niloticus: Biochemical and oxidative stress. J Basic Appl Zool 72:43–57
Adeel M, Ma C, Ullah S, Rizwan M, Hao Y, Chen C, Jilani G, Shakoor N, Li M, Wang L, Tsang DCW, Rinklebe J, Rui Y, Xing B (2019) Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural and oxidative damage: a life cycle study on Eisenia fetida. Environ Pollut 254(2019):113032
Adhikari S, Sarkar B (2004) Effects of cypermethrin and carbofuran on certain haematological parameters and prediction of their recovery in fresh water teleost, Labeo rohita (Ham). Ecotoxicol Environ Saf 58:220–226
Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 56:300–306
Ale A, Bacchetta C, Rossi AS, Galdopórpora J, Desimone MF, de la Torre FR, Gervasio S, Cazenave J (2018) Nano silver toxicity in gills of a neotropical fish: metal accumulation, oxidative stress, histopathology and other physiological effects. Ecotoxicol Environ Saf 148:976–984. https://doi.org/10.1016/j.ecoenv.2017.11.072
Alkaladi A, El-Deen N, Afifi M, Abu Zinadah O (2015) Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J Biol Sci 22:556–563
Anand SR, Mathan R, Manoharan S, Rama KP, Subramanian B, Devaraj N (2015) Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on haematology, iono regulation and gill Na+/K+ ATPase activity. J King S Uni - Sci 27:151–160
Anbumani S, Mohankumar MN (2012) Gamma radiation induced micronuclei and erythrocyte cellular abnormalities in the fish Catla catla. Aquat Toxicol 122–123:125–132
Atli G, Ariyurek SY, Kanak EG, Canli M (2015) Alterations in the serum biomarkers belonging to different metabolic systems of fish (Oreochromis niloticus) after Cd and Pb exposures. Environ Toxicol Pharmacol 40(2):508–515
Baek YW, An YJ (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ 409:1603–1608
Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2011) Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pestic Biochem Physiol 99:1–6. https://doi.org/10.1016/J.PESTBP.2010.09.001
Bayunova L, Barannikova I, Semenkova T (2002) Sturgeon stress reactions in aquaculture. J Appl Ichthyol 18:397–404. https://doi.org/10.1046/j.1439-0426.2002.00410.x
Bernet D, Schmidt H, Wahli T, Burkhardt-Holm P (2000) Effluent from a sewage treatment works causes changes in serum chemistry of brown trout (Salmo trutta L.). Ecotoxicol Environ Saf 48:140–147
Bilberg K, Malte H, Wang T, Baatrup E (2010) Silver nanoparticles and silver nitrate cause respiratory stress in Eurasian perch (Perca fluviatilis). Aquat Toxicol 96:159–165. https://doi.org/10.1016/j.aquatox.2009.10.019
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781
Bowers LD, Edward TW (1980) Kinetic serum creatinine assays. II. A critical evaluation and review. Clin Chem 26(5):555–561
Brand ME (2001) Bioaccumulation of metals in Labeo congoro from the Olifants River (Mpumalanga) and the effect of nickel on the haematology of fish (M.Sc. Sci. thesis). Rand Afrikaans Univ
Brody AL (2006) Nano and food packaging technologies converge. Food Technol 60:92–94
Bystrzejewska-Piotrowska G, Golimowski J, Urban PL (2009) Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag 29(9):2587–2595. https://doi.org/10.1016/j.wasman.2009.04.001
Canli EG, Canli M (2015) Low water conductivity increases the effects of copper on the serum parameters in fish (Oreochromis niloticus). Environ Toxicol Pharmacol 39(2):606–613
Canli EG, Atli G, Canli M (2017) Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ Toxicol Pharmacol 50:145–150
Canli EG, Dogan A, Canli M (2018) Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ Toxicol Pharmacol 62:181–187. https://doi.org/10.1016/j.etap.2018.07.009
Carbis CR, Mitchell GF, Anderson JW, McCauley I (1996) The effects of microcystins on the serum biochemistry of carp, Cyprinus carpio L., when the toxins are administered by gavage, immersion and intraperitoneal routes. J Fish Dis 19:151–159
Cavas T, Garanko NN, Arkhipchuk VV (2005) Induction of micronuclei and binuclei in blood, gill and liver cells of fishes chronically exposed to cadmium chloride and copper sulphate. Food Chem Toxicol 43:569–574
Cheraghi J, Hosseini E, Hoshmandfar R, Sahraei R (2013) Hematologic parameters study of male and female rats administrated with different concentrations of silver nanoparticles. Int J Agric and J Crop Sci 5:789–796
Clark NJ, Shaw BJ, Handy RD (2018) Low hazard of silver nanoparticles and silver nitrate to the haematopoietic system of rainbow trout. Ecotoxicol Environ Saf 152:121–131. https://doi.org/10.1016/j.ecoenv.2018.01.030
Coles EH (1986) Veterinary clinical pathology (4th Ed); W. B. Saunders Co, Philadelphia
Corcoran RM, Durnan SM (1977) Albumin determination by a modified bromocresol green method. Clin Chem 23(4):765–766
Cui B, Ren L, Xu QH, Yin LY, Zhou XY, Liu JX (2016) Silver nanoparticles inhibited erythrogenesis during zebrafish embryogenesis. Aquat Toxicol 177:295–305. https://doi.org/10.1016/j.aquatox.2016.06.005
Dacie JA, Lewis SM (1991) Practical haematology A, 7th edn. Churchill Livingstone, London
Dumala N, Mangalampalli B, Kalyan Kamal SS, Grover P (2019) Repeated oral dose toxicity study of nickel oxide nanoparticles in Wistar rats: a histological and biochemical perspective. J Appl Toxicol 1–18:1012–1029. https://doi.org/10.1002/jat.3790
Evans DW, Dodoo DK, Hanson PJ (1993) Trace element concentrations in fish livers: implications of variations with fish size in pollution monitoring. Mar Pollut Bull 26:329–334. https://doi.org/10.1016/0025-326X(93)90576-6
Firat Ö, Kargin F (2010) Individual and combined effects of heavy metals on serum biochemistry of Nile Tilapia, Oreochromis niloticus. ArchEnviron Contam Toxicol 58:151–157
Farkas J, Farkas P, Hyde D (2004) Liver and gastroenterology tests. In: Lee M 3rd (ed) Basic skills in interpreting laboratory data. American Society of Health-System Pharmacists, Bethesda, pp 330–336
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500:237–242. https://doi.org/10.1016/j.aquaculture.2018.10.030
Freitas RA (2005) What is nanomedicine? Nanomedicine 1:2–9
George S, Gardner H, Seng EK, Chang H, Wang C, Yu Fang CH, Richards M, Valiyaveettil S, Chan WK (2014) Differential effect of solar light in increasing the toxicity of silver and titanium dioxide nanoparticles to a fish cell line and zebrafish embryos. Environ Sci Technol 48:6374–6382. https://doi.org/10.1021/es405768n
Ghafari FH, Binde DH, Jamali H, Hasanpour S, Mehdipour N, Rashidiyan G (2017) The protective role of vitamin E on Oreochromis niloticus exposed to ZnONP. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2017.07.005
Ghiasi F, Mirzargar SS, Badakhshan H, Shamsi S (2010) Effects of low concentration of cadmium on the level of lysozyme in serum, leukocyte count and phagocytic index in Cyprinus carpio under the wintering conditions. J Fish Aquat Sci 5:113–119. https://doi.org/10.3923/jfas.2010.113.119
Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y (2011) Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 83:510–516
Grosell M, McDonald MD, Wood CM, Walsh PJ (2004) Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta) I: hydromineral balance and plasma nitrogenous waste products. Aquat Toxicol 68:249–262
Gupta US, Guha S (2006) Microcystin toxicity in a freshwater fish, Heteropneustes fossilis (Bloch). Curr Sci 91:9–10
Hajirezaee S, Mohammadi G, Naserabad SS (2019) The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2- NPs). Aquaculture 518:734734. https://doi.org/10.1016/j.aquaculture.2019.734734
Han ZX, Zhang M, Xia LC (2012) Bioaccumulation and toxicity of NiO nanoparticles in Gracilaria lemaneiformis. Adv Mater Res 518–523:942–945
Handy RD, Kammer FVD, Lead JR, Hassellöv M, Owen R, Crane M (2008) The ecotoxicity and chemistry of manufactured nanoparticles. Ecotoxicology 17:287–314
Hedayati SA, Farsani HG, Naserabad SS, Hoseinifar SH, Van Doan H (2019) Protective effect of dietary vitamin E on immunological and biochemical induction through silver nanoparticles (AgNPs) inclusion in diet and silver salt (AgNO3) exposure on Zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 222:100–107. https://doi.org/10.1016/j.cbpc.2019.04.004
Hontela A, Rasmussen JB, Audet C, Chevalier G (1992) Impaired cortisol stress response in fish from environments polluted by PAHs, PCBs, and mercury. Arch Environ Contam Toxicol 22:278–283. https://doi.org/10.1007/BF00212086
Horie M, Fukui H, Nishoi K, Endoh S, Kato H, Fujita K, Miyauchi A, Nakamura SM, Ishida N, Kinugas S, Morimoto Y, Niki N, Yoshida Y, Iwahashi H (2011) Evaluation of acute oxidative stress induced by NiO nanoparticles in vivo and in vitro. J Occup Health 53:64–74. https://doi.org/10.7897/2230-8407.0910238
Imani M, Halimi M, Khara H (2015) Effects of silver nanoparticles (AgNP) on haematological parameters of rainbow trout, Oncorhynchus mykiss. Comp Clin Pathol 24(3):491–495. https://doi.org/10.1007/s00580-014-1927-5
Javed M, Usmani N (2013) Investigation on accumulation of toxicants and health status of freshwater fish Channa punctatus, exposed to sugar mill effluent. Int J Zool 3(1):43–48
Jendrassik L, Grof P (1938) Colorimetric method of determination of bilirubin. Biochem Z 297:81–82
Jindal R, Kaur M (2014) Phenotypic alterations in erythrocytes of Ctenopharyngodon idellus (Cuvier & Valenciennes) induced by chlorpyrifos: SEM Study. Int J of Fish and Aqua Sci 4(1):23–30
Johansen K (1970) Air-breathing fishes. In: Hoar WS, Randall DT (eds) Fish physiology, vol 4. Academic, New York, pp 361–411
Jorgensen SW (2010) A derivative of encyclopaedia of ecology. Ecotoxicology. Academic Press, London, p 390
Kandeel NMS (2004) Toxicological and metabolic studies of some molluscicides on harmful terrestrial snails (M.Sc. thesis). Zoology Dep., Faculty of Science, Cairo University
Karnik BS, Davies SH, Baumann MJ, Masten SJ (2005) Fabrication of catalytic membranes for the treatment of drinking water using combined ozonation and ultrafiltration. Environ Sci Technol 39:7656–7661
Kaur A, Kaur K (2006) Impact of nickel-chrome electroplating effluent on the protein and cholesterol contents of blood plasma of Channa punctatus (Bl.) during different phases of the reproductive cycle. J Environ Biol 27(2):241–245
Kaviani EF, Naeemi AS, Salehzadeh A (2019) Influence of copper oxide nanoparticle on haematology and plasma biochemistry of Caspian trout (Salmo trutta caspius), following acute and chronic exposure. Pollution 5(1):225–234. https://doi.org/10.22059/poll.2018.251034.383
Kaviani EF, Naeemi AS, Salehzadeh A (2020) Acute toxicity and effects of titanium dioxide nanoparticles (TiO2 NPs) on some metabolic enzymes and hematological indices of the endangered Caspian trout juveniles (Salmo trutta caspius Kessler, 1877). Iran J Fish Sci 19(3):1253–1267
Khan MS, Qureshi NA, Jabeen F (2017a) Assessment of toxicity in fresh water fish Labeo rohita treated with silver nanoparticles. Appl Nanosci. https://doi.org/10.1007/s13204-017-0559-x
Khan MS, Qureshi NA, Jabeen F, Shakeel M, Asghar MS (2017c) Assessment of waterborne amine-coated silver nanoparticle (Ag-NP)-induced toxicity in Labeo rohita by histological and hematological profiles. Biol Trace Elem Res 182:1–10. https://doi.org/10.1007/s12011-017-1080-5
Khosravi-Katuli K, Lofrano G, Pak Nezhad H, Giorgio A, Guida M, Aliberti F, Siciliano A, Carotenuto M, Galdiero E, Rahimi E, Libralato G (2018) Effects of ZnO nanoparticles in the Caspian roach (Rutilus rutilus caspicus). Sci Total Environ 626:30–41
Kind PR, King EG (1954) Colorimetric determination of alkaline phosphatase activity. J Clin Pathol 7:322–326
Kovrižnych JA, Sotníková R, Zeljenková D, Rollerová E, Szabová E, Wimmerová S (2013) Acute toxicity of 31 different nanoparticles to zebra fish (Danio rerio) tested in adulthood and in early life stages – comparative study. Interdiscip Toxicol 6:67–73
Kumar PYG, Gupta VT, Shakti T, Singh A (2005) Haematological and biochemical abnormalities in Cirrhinus mrigala (Ham.) induced by lead. J Ecophysiol Occupl Hlth 5:213–216
Kumar SM, KP, Ramesh M (2011) Haematological and biochemical responses of fresh water teleost fish; Cyprinus carpio (Actinopterygii: Cyprinusformes) during acute and chronic sublethal exposure to lindane. Pest Biochem Physiol 100: 206-211
Lanone S, Boczkowski J (2006) Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr Mol Med 6:651–663
Larsson A, Haux C, Sjobeck M (1985) Fish physiology and metal pollution. Results and experiences from laboratory and field studies. Ecotoxicol Environ Saf 9:25–281
Lasheen MR, AbdelGawad FK, Alaneny AA, Abdelbary HMH (2012) Fish as Bio indicators in aquatic environmental pollution assessment: a case study in Abu-Rawash Area, Egypt. World Appl Sci J 19:265–275
Lowry OH, Rosenbrough NJ, Ferry AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Luoma SN, Rainbow PS (2008) Sources and cycles of trace metals. In: Metal contamination in aquatic environments: science and lateral management. Cambridge University Press, Cambridge, pp 47–66
Maheswaran R, Devapaul A, Velmurugan B, Ignacimuthu S (2008) Haematological studies of freshwater fish, Clarias batrachus (L.) exposed to mercuric chloride. Int J Integr Biol 2(1):49–54
Mahjoubian M, Naeemi AS, Sheykhan M (2021) Toxicological effects of Ag2O and Ag2CO3 doped TiO2 nanoparticles and pure TiO2 particles on zebrafish (Danio rerio). Chemos. 263:128182
Maita M, Shiomitsu L, Ikeda Y (1985) Health assessment by the climogram of hemochemical constituents in cultured yellowtail. Bull Jpn Soc 51: 205–211
Massarsky A, Abraham A, Nguyen KC, Rippstein P, Tayabali AF, Trudeau VL, Moon TW (2014) Nanosilver cytotoxicity in rainbow trout (Oncorhynchus mykiss) erythrocytes and hepatocytes. Comp Biochem Physiol Part C: Toxicol Pharmacol 159:10–21. https://doi.org/10.1016/j.cbpc.2013.09.008
Molina R, Moreno I, Pichardo S, Jos A, Moyano R, Monterde JG, Cameán A (2005) Acid and alkaline phosphatase activities and pathological changes induced in Tilapia fish (Oreochromis sp.) exposed sub-chronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon. https://doi.org/10.1016/j.toxicon.2005.07.012
Monteiro D, Rantin F, Kalinin A (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19:105–123
Munkittrick KR, Leatherland JF (1983) Haematocrit values in feral goldfish, Carassius auratus L., as indicators of the health of the population. J. Fish Biol 23:153–161
Naeemi AS, Elmi F, Vaezi G, Ghorbankhah M (2020) Copper oxide nanoparticles induce oxidative stress mediated apoptosis in carp (Cyprinus carpio) larva. Gene Repor 19:100676. https://doi.org/10.1016/j.genrep.2020.100676
National Science and Technology Council (2004) National Nanotechnology Initiative Strategic Plan; Executive Office of the President of the United States: Washington, DC, US
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding bio physicochemical interactions at the nano-bio interface. Nat Mater 8(7):543–557
Ogunola OS (2017) Physiological, immunological, genotoxic and histopathological biomarker responses of molluscs to heavy metal and water-quality parameter exposures: a critical review. J Oceanogr Mar Res 5:158. https://doi.org/10.4172/2572-3103.1000158
Oner M, Atli G, Canli M (2008) Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal, (Ag, Cd, Cr, Cu, Zn) exposures. Environ Toxicol Chem 27:360–366
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haematology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci 6(1):19–29
Rao KV, Sunandana CS (2008) Effect of fuel to oxidizer ratio on the structure, micro structure and EPR of combustion synthesized NiO nanoparticles. J Nanosci Nanotechnol 8:4247–4253
Razavian MH, Masaimanesh M (2015) Ingestion of silver nanoparticles leads to changes in blood parameters. Nanomed J 1(5):339–345
Reitman S, Frankel SA (1957) Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic-pyruvic transaminase. Am J Clin Pathol 28–56
Remya AS, Ramesh M, Saravanan M, Poopal RK, Bharathi S, Nataraj D (2015) Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on hematology, iono regulation and gill Na+/K+ ATPase activity. Journal of King Saud University – Science 27(2):151–160
Roco MC (2003) Nanotechnology convergence with modern biology and medicine. Curr Opin Biotechnol 14:337–346
Sadoul B, Vijayan MM (2016) 5 - stress and growth. In: Schreck LT, Farrell AP, Colin J, Brauner Carl B (eds) Fish physiology, Biology of Stress in Fish. Academic Press, pp 167–205
Said REM, Hasieb HE, Moustafa MA, Soliman SME, Klaos W, Osman GM (2019) Haematological, serological and genotoxic findings in the African catfish Clarias gariepinus after the administration of copper nanoparticles and penconazole. EC Veterinary Science 4(10):01–14
Salimi A, Sharifi E, Noorbakhsh A, Soltanian S (2007) Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nano-scale islands of nickel oxide. Biophys Chem 125:540–548
Samuel RM (1986) Haematology. In: Notes on clinical lab techniques, 4th edn. Tailor, Madras
Sara V, Ghasem M, Kamran RT, Fatemeh M, Saeid SN (2020) The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): bioaccumulation, haematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxi Environ Safety 194:110353. https://doi.org/10.1016/j.ecoenv.2020.110353
Saravanan M, Kumar, KP, Ramesh M (2011) Haematological and biochemical responses of fresh water teleost fish; Cyprinus carpio (Actinopterygii: Cyprinusformes) during acute and chronic sublethal exposure to lindane. Pest Biochem Physiol 100: 206–211
Saunders DC (1967) Differential blood cell counts of 121 species of marine fishes of Puerto Rico. Trans Am Microsc Soc 85:427–449
Sayed AEDH, Kataoka C, Oda S, Kashiwada S, Mitani H (2018) Sensitivity of medaka (Oryzias latipes) to 4-nonylphenol subacute exposure; erythrocyte alterations and apoptosis. Environ Toxicol Pharmacol 58:98–104. https://doi.org/10.1016/j.etap.2017.12.023
Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF (2010) Metal-based nanoparticles and their toxicity assessment. Wiley Interdisciplinary Reviews Nanomed Nanobiotech 2:544–568
Shah N, Khisroon M, Ali Shah SS (2020) Assessment of copper, chromium, and lead toxicity in fish (Ctenopharyngodon idella Valenciennes, 1844) through hematological biomarkers. Environ Sci Pollut Res 27:33259–33269
Shaluei F, Hedayati A, Jahanbakhshi A, Kolangi H, Fotovat M (2013) Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Human Exp Toxicol 32:1270–1277.
Sheeba AS, Noorjahan CM (2018) Toxicity of copper nanoparticle on haematology and biochemistry of fish, Tilapia mossambica. Int Res J Pharm 9(10):121–124
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10:15. https://doi.org/10.1186/1743-8977-10-15
Sookoian S, Pirola CJ (2012) Alanine and aspartate aminotransferase and glutamine cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 18:3775–3781. https://doi.org/10.3748/wjg.v18.i29.3775
Svoboda M, Luskova V, Drastichova J, Iabek V (2001) The effect of diazinon on hematological indices of common carp (Cyprinus carpio L.). Acta Vet (Brno) 70:457–465
Svobodova Z (1971) Some hematological and metabolic changes in fish occurring after pesticide intoxication. Bull VUR Vodany 7:29–36
Thummabancha K, Onparn N, Srisapoome P (2016) Molecular characterization and expression analyses of cDNAs encoding the thioredoxin-interact-ing protein and selenoprotein P genes and histological changes in Nile tilapia (Oreochromis niloticus) in response to silver nanoparticle exposure. Gene. 577:161–173
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35:1366–1375. https://doi.org/10.1016/j.dci.2011.07.002
Trinder P (1969) Enzymatic colorimetric method of glucose. Ann Clin Biochem 6:24–27
Van der OR, Beyer J, Vermeulen NPEE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Vaseem H, Banerjee TK (2012) Toxicity analysis of effluent released during recovery of metals from polymetallic sea nodules using fish haematological parameters. In: Ali M (ed) The functioning of ecosystem. Intech, Croatia, pp 249–260
Vidya PV, Chitra KC (2018) Evaluation of genetic damage in Oreochromis mossambicus exposed to selected nanoparticles by using micronucleus and comet bioassays. Croatian Journal of Fisheries 76:115–124. https://doi.org/10.2478/cjf-2018-0015
Voet F, Voet JG (1990) Biochemistry. John Wiley and Sons, New York, USA, pp 425–457
Vosyliene MZ (1999) The effect of heavy metal mixture on haematological parameters of rainbow trout. Heavy metals in environment. An integrated approach Ed. Lovejy DA. 295-298
Wang J, Zhu X, Zhang X, Zhao Z, Liu H, George R, Wilson-Rawls J, Chang Y, Chen Y (2011) Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemos. 83(4):461–467
Weishaar D, Gossrou E, Faderl B (1975) Ranges of alpha-HBDH, LDH, AP and LAP as measured with substrate optimated test charges. Med Welt 26:387–392
Wood CM, Farrel AP, Brauner CJ (2012a) Homeostasis and toxicology of essential metals. Fish Physiol. 31A Academic Press, London, pp. 497
Wood CM, Farrel AP, Brauner CJ (2012b) Homeostasis and toxicology of non-essential metals. Fish Physiol. 31B Academic Press, London, pp. 507
Young G, Brown CL, Nishioka RS, Folmar LC, Andrews M, Cashman JR, Bern HA (1994) Histopathology, blood chemistry and physiological status of normal and moribund striped bass (Morone saxatilis) involved in summer mortality (‘die-off’) in the Sacramento-San Joaquin Delta of California. J Fish Biol 44:491–512
Zaghloul KH, Omar WA, Abdo-Hegab S (2006) Toxicity specificity of copper in some freshwater fishes. Egypt J Zool 47:383–400
Zhang Y, Zhu L, Zhou Y, Chen J (2015) Accumulation and elimination of iron oxide nanomaterials in zebrafish (Danio rerio) upon chronic aqueous exposure. J Environ Sci 30:223–230
Zutshi BSG, Prasad R, Nagaraja R (2010) Alteration in hematology of Labeo rohita under stress of pollution from Lakes of Bangalore, Karnataka, India. Environ Monit Assess 168(1-4):11–19
Acknowledgements
The authors duly acknowledge Analytical Discipline and Centralized Instrument Facility of Aligarh Muslim University for providing instrumental facilities. The authors also acknowledge Prof. Iqbal Parvez for providing microscope facility of Department of Zoology, Aligarh Muslim University.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study was financially supported by University Grant Commission (UGC), by the grant of STARTUP (No. F.30-409/2018(BSR)), UGC, Government of India, New Delhi, India.
Author information
Authors and Affiliations
Contributions
A.R Samim has performed experiments, analysed data and prepared manuscript. H. Vaseem has designed and coordinated the experiments, interpreted the results and improved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samim, A.R., Vaseem, H. Assessment of the potential threat of nickel(II) oxide nanoparticles to fish Heteropneustes fossilis associated with the changes in haematological, biochemical and enzymological parameters. Environ Sci Pollut Res 28, 54630–54646 (2021). https://doi.org/10.1007/s11356-021-14451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14451-y