Abstract
This research was intended to evaluate the antidiabetic effect of single or combined administration of nanoparticles of zinc oxide nanoparticles (ZnONPs), chromium oxide nanoparticles (Cr2O3NPs), and selenium nanoparticles (SeNPs), on genetic and metabolic insult in fructose/streptozotocin diabetic rat model. Type 2 diabetes mellitus was induced by feeding sixty adult male albino rats with a high fructose diet accompanied by a single i.p. injection of streptozotocin (STZ). The rats were divided into 6 groups (10 rats/each) and the doses of nanoparticles were 10 mg/kg b.wt for ZnONPs, 1 mg/kg b.wt for Cr2O3, and 0.4 mg/kg b.wt for SeNPs. The results displayed that diabetes significantly decreased bodyweight, serum insulin, C-peptide, adiponectin levels, erythrocyte glutathione peroxidase, serum superoxide dismutase activities, high-density lipoprotein cholesterol (HDL-C), and total antioxidant capacity while causing a substantial increase in serum glucose, C-reactive protein, atherogenic index, HOMA–IR, malondialdehyde, lipid profile, interleukin-6 levels, and liver function and kidney function parameters. Furthermore, the findings showed a decrease in insulin receptor substrate-1 (IRS-1) hepatic mRNA expression level and peroxisome proliferator-activated receptor (PPAR-γ) adipocyte mRNA expression level in type 2 diabetic rats. DNA damage was confirmed by performing the comet assay. Moreover, histological observation of pancreatic and hepatic tissues was performed, which were consistent with the biochemical results. The present study confirmed that oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture improved all the biochemical and genetic parameters toward normal levels and ameliorated the diabetic consequences that were manifested by restricting cellular DNA damage which maintaining pancreatic and hepatic tissues from oxidative damage. The best reported antidiabetic effect was observed in the mixture administered group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a chronic progressive metabolic disorder and a multifactorial disease associated with many pathological alterations including microvascular and macrovascular complications that affect almost every part of the body (Othman et al. 2019). Diabetes mellitus causes chronic disorders that affect the metabolism of carbohydrates, fats, proteins, and electrolytes in the body, leading to severe complications which are classified into acute, sub-acute, and chronic. Acute complications include hypoglycemia, diabetic ketoacidosis, hyperosmolar, and hyperglycaemic non-ketotic syndrome, while sub-acute complications include thirst, polyuria, lack of energy, visual blurriness, and weight loss (Wild et al. 2004). Based on the World Health Organization 2014, there are currently > 400 million people suffering from diabetes worldwide, and that number will reach to 552 million by 2030 (WHO.2014), 90% of those with diabetes have type 2 diabetes (T2DM). This type of diabetes is a complex and heterogeneous disorder that is distinguished by a progressive decline in insulin action or defect in insulin signal transduction (Jayawardena et al. 2012). It is strongly correlated with obesity and insulin resistance and defects in pancreatic β-cell function and mass (Afifi et al.2020). However, it takes years to develop T2DM.Type 2 diabetes patients have also experienced obesity associated with decreased insulin sensitivity in parallel with an active β-cell compensatory system, such as extra basal insulin secretion and hyper-pro-insulinemia as part of their metabolic profile. For the induction of either type 1 or type 2 diabetes, STZ has been used alone or in combination with other chemicals or with dietary manipulations. Fructose intake in excess can induce moderate obesity and have several deleterious metabolic effects, including hypertriglyceridemia and hyperinsulinemia (Elankani et al. 2020).
Nanotechnology is a fast-growing research domain that has led to huge significant scientific breakthroughs. It includes the formation and control of particles at “nanoscale” having novel and improved physicochemical properties. Recent study has shown that mineral nanoparticles have greater bioavailability due to their new characteristics, such as greater specific surface area, high catalytic efficiency, higher surface activity, and stronger adsorbing ability, as well as it may be readily carried up by the gastrointestinal tract and used in the animal system and reached to deeper tissues by high efficient than the larger-sized particles (Tag-ElDin 2019).
Many researches demonstrated the role of metals in glucose metabolism and the association of their deficiency with diabetes as vanadium (Thompson et al. 2009), chromium (Wang and Cefalu 2010), and zinc (Chausmer 1998) has been reported to play a role in blood sugar maintenance and has been included in diabetes therapy. Zinc (Zn) is an essential micronutrient for pancreatic function through its effect on insulin stability and disturbance in Zn metabolism associated with diabetic complications; zinc promotes hepatic glycogenesis through its actions on the insulin pathways and thus improves glucose utilization (Jansen et al. 2009). Zinc has a role in insulin biosynthesis, storage, and secretion. There are several zinc transporters in pancreatic β-cells (Smidt et al. 2009), like zinc transporter 8 which has a potent role in insulin secretion (Rungby 2010). In the medicine field, zinc oxide nanoparticles (ZnONPs) have demonstrated great challenges. The antidiabetic activity of ZnONPs (as a novel agent to regulate diabetes mellitus in rats) was proven in a previous study (Abd El-Rahman et al. 2016). Umrani and Paknikar 2014 have reported significantly anti-diabetic effects of oral treatment of zinc oxide nanoparticles which have enhanced glucose tolerance. In diabetic rats, blood glucose levels were reduced, insulin levels and expression were increased, glucokinase (GK) activity was increased, and insulinreceptor substrates (IRS) and glucose transporter 2 (GLUT-2) expression levels were improved. Chromium is an essential nutrient required for optimal insulin activity as well as normal carbohydrate and lipid metabolism (Anderson 1998). Accumulating evidence showed that tissue chromium levels of subjects with diabetes are lower than those of normal control subjects, and a correlation exists between low circulating levels of chromium and the incidence of type 2 diabetes (Anderson et al. 1991). It has been proposed that chromium may increase the number of insulin receptor substrates, enhance receptor binding, and enhance intracellular signaling leading to the improvement of insulin resistance (Chen et al. 2009). Some studies have found that chromium supplementation significantly decreased the fasting blood glucose and decreased the HbA1c of diabetic individuals (Anderson 1998; Ghosh et al. 2002). Chromium-deficient diets are associated with impaired glucose tolerance, hyperglycemia, and hyperinsulinemia (Abdourahman and Edwards 2008). Chromium oxide nanoparticles (Cr2O3NPs) could improve the cellular antioxidant capacity, so, its supplementation is an effective treatment strategy to minimize increased oxidative stress in type 2 diabetic patients (Berenjian et al. 2018). It is important to remember that one of the first and few studies examining the use of chromium oxide in the form of nanoparticles is our research. Selenium (Se) is an important element for health with important biological and biochemical functions due to its antioxidant properties (Chen et al. 2006). Se is a basic component of selenoprotein which reduces oxidative stress (Ahmed et al. 2017). The relation between selenium and T2DM is controversial (Wang et al. 2016). Thus, untreated diabetes causes oxidative stress which leads to complications of the disease, and the antioxidant intake is considered being favorable for the therapy of diabetes (Mueller et al. 2009), Selenium plays a protective role against T2DM (Steinbrenner and Sies 2009). Selenium nanoparticles (SeNPs) have gained considerable attention due to their biological activities and biocompatibility. As a food additive, SeNPs below 100 nanometers have a high functioning potential and antioxidant properties that are useful for human health. As antioxidants, selenium nanoparticles have less toxicity compared with selenium in their bulk form. SeNPs have been considered a promising instrument in drug therapies for type 2 diabetes; this is due to their high therapeutic properties and low toxicity (Srivastava et al. 2014). The aim of the current research was to evaluate the impact of single or combined administration of nanoparticles of zinc oxide, chromium oxide, and selenium on genotoxicity and metabolic consequences in type 2 diabetic rat model.
Materials and methods
Synthesis of nanoparticles
Synthesis of zinc oxide nanoparticles (ZnONPs)
ZnO nanoparticles were prepared via co-precipitation method (Mohamed and Attia 2020) by adding 50 ml NaOH solution (4 M) into 50 ml of ZnSO4 solution (0.2 M) at an approximate rate of 5 ml/min under vigorous stirring and the stirring was continued for 12 h. Then, the collected precipitate was filtered, washed three times with deionized water, and dried at 100 °C in an oven. The collected powder was calcined for 2 h at 500 °C.
Synthesis of chromium oxide nanoparticles (Cr2O3NPs)
A solution of 10 ml of 0.2M of K2Cr2O7 was added to a solution of 5 ml of 0.96g/ml of cetyltrimethyl ammonium chloride (CTAC) and 50ml of 10mM of trisodium citrate. The vessel reaction was illuminated to halogen lamp (HALOPAR 20 75 W 230 V 30° GU10, Italy) for 3 h under stirring. The solution color was changed from brownish-yellow to green, which showed Cr2O3 nanoparticles being formed (Attia et al.2020).
Synthesis of selenium nanoparticles (SeNPs)
The wet chemical reduction method was modified to synthesis SeNPs (Emam et al. 2020). In this method, 2.5ml of 0.2M of cetyltrimethyl ammonium chloride (CTAC) solution was added to a solution of 0.07 M ascorbic acid (40ml) under stirring. Then after 5 min of stirring, 1ml of 0.25M of sodium selenite was added drop-wise till the solution color changed from colorless into orange color.
Nanoparticles characterization
UV–Vis spectra were reported using a Hewlett-Packard 8452A diode-array spectrophotometer. Measurements of X-ray diffraction (XRD) were recorded by using a Philips PW1710 X-ray diffractometer (k = 1.54186 A°) from 20° to 70° 2H, with a step size of 0.020° 2H and collecting 10s per step. The size and shape of the formed nanoparticles were performed using transmission electron microscopy (TEM-JEOL JEM-2100 of 200 kV with a magnification range of ×1000 to ×50,000).
Animals
Sixty adult male albino rats of Sprague -Dawley strain, weighing 120±5g of 6 weeks’ age were used in the present study. Animals were obtained from Breeding Unit from Ophthalmology Research Institute (Giza, Egypt).
Diets
The standard diet was prepared to meet the rat’s nutrient requirements according to the American Institute of Nutrition (AIN-93Maintenance diet) and adjusted by Reeves et al. 1993. A high fructose diet (HF) was prepared by modifying diet materials for the induction of insulin resistance by adding 21% fructose to the diet according to Kumar et al. 2014.
Induction of type2 diabetes mellitus
For induction of type 2 diabetes mellitus, group of 50 rats received a high fructose diet for induction of insulin resistance that was prepared by modifying diet materials by adding 21% fructose for 4 weeks as described by Kumar et al. 2014. After these 4 weeks, and 12-h fasting, this group was intraperitoneally (i.p.) injected by freshly prepared STZ solution in a dose of 40 mg/kg body weight (Kumar et al .2014). STZ was freshly prepared in 0.1M citrate phosphate buffer (pH 4.5). The blood glucose random levels were tracked afterwards. The glucose concentration in blood was taken from the tail vein and was determined by the enzymatic colorimetric method (Trinder 1969). Stable blood glucose levels higher than 200 mg/dl were considered to indicate diabetes onset (Gandhi and Sasikumar 2012). Also, group of normal control in the same time was intraperitoneally (i.p.) injected by saline.
Experimental design
The experimental study was performed on 60 rats divided into 6 groups (n = 10): Group I served as control: this group underwent normal diet and water and received daily oral doses of saline; Group 2, Diabetic rats: rats that fulfilled the previously mentioned criteria; Group 3: diabetic rats received daily oral doses of ZnONPs (10 mg/kg b.wt) according to Umrani and Paknikar.2014; Group 4: diabetic rats received daily oral doses of Cr2O3NPs (1mg/kg b.wt) according to Abou Zaid et al.2015; Group 5: diabetic rats received daily oral doses of SeNPs (0.4mg/kg b.wt) according to Rezaei- Kelishadi et al.(2017); and Group 6: diabetic rats received daily oral doses of a mixture of nanoparticles (ZnONPs+Cr2O3NPs+SeNPs) in a ratio1:1:1 by mixing equal volume from each solution of them. The oral doses of several treatments were given daily using intragastric intubation (p.o.). During the experimental approach (6 weeks), a food intake was reported daily while the bodyweight of rats was tracked weekly.
Food intake was recorded daily for each group of rats by subtracting the residue and refusal diet from supplied diet.
In body weight change, rats were weighted weekly to monitor body weight change that was calculated by subtracting initial body weight from final body weight.
Sample preparation
At the end of six weeks and after 8hrs fasting from (11 pm to 7 am) blood samples were taken from the tail vein for determination of glucose concentration by the enzymatic colorimetric method as described by Trinder 1969. Four hours later (completing 12 h fasting) the animals were sacrificed under isoflurane anesthesia.
Blood samples were collected directly from the hepatic portal vein, in two centrifuge tubes; the first tube was containing EDTA to collect whole blood used for HbA1C, and erythrocyte glutathione peroxidase (GPx) activity determination. The second test tube was used for separation of serum by allowing blood samples, left for 15 min at 37°C then centrifuged at 4000 rpm for 20 minutes. Serum samples were kept in plastic vials and stored in a deep freezer at -20 °C until used for other biochemical analyses. Liver and adipose tissue (visceral) specimens were washed twice and bottled on filter papers then kept in QiaZol (QiagenInc., Valencia, CA, USA) at -80°C until used for molecular assays. Pancreas and part of liver tissues were preserved in 10% formalin solution for histological examination.
Biochemical analyses
Serum analyses
The enzyme colorimetric approach used the Salucea kit, Netherlands Trinder 1969. It has been used to measure the serum glucose level. Serum insulin level was measured by rat insulin ELISA Millipore kit as described by Vikram et al. 2011. The homeostasis model assessment of basal insulin resistance (HOMA-IR) was calculated according to the method of Matthews et al. 1985. Serum proinsulin C-peptide and adiponectin levels were measured using ELISA kit as described by O’Rahilly and Moller 1992 and Shihabudeen et al. 2015, respectively. Glycosylated hemoglobin (HbA1c) was determined by the colorimetric method using the Elabscience kit as described by Nathan et al. 1984.
Erythrocyte glutathione peroxidase (GPX) activity was determined as described by Ursini et al. 1985 using the Cayman kit, USA. Serum superoxide dismutase (SOD) activity was determined using the ELISA technique (EIAab ELISA kit) as described by McCord and Fridovich 198). Total antioxidant capacity (TAC) was determined as described by Trachootham et al. 2008 using OxiSelect kits (USA). Nitric oxide (NO) levels and serum malondialdehyde (MDA) were determined by the colorimetric method using the Sigma-Aldrich kit as described by Bulau et al. 2007 and Ohkawa et al. 1979, respectively. Serum C-reactive protein (CRP) and interleukin 6 (IL-6) levels were measured following the ELISA technique for rats as described by Ronald 1997 and Hirano 1998 using Cusabio and Biovision kits (USA), respectively.
High-density lipoprotein cholesterol (HDL-C), triacylglycerol (TAGs), and serum total cholesterol were determined following the colorimetric methods as described by Lopes-Virella et al. 1977, Fassati and Prencipe 1982, and Allain et al. 1974, respectively. Serum very low-density lipoprotein cholesterol (VLDL-C) and low-density lipoprotein cholesterol (LDL-C) concentration were calculated according to Friedewald et al. 1972. While the atherogenic coefficient and atherogenic index risk ratio were calculated as described by Brehm et al. 2004 and Dobiásová and Frohlich 2001.
Colorimetric approach was used for the measurements of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as described by Reitman and Frankel 1957. The colorimetric method described by Doumas et al. 1971 was used to measure serum albumin. The enzymatic kinetic method described by Fawcett and Scott 1960 was also used to determine serum urea and serum creatinine was determined by the colorimetric method as described by Butler 1975 using the Elitech kit (France). Detection limits of ELISA were 15.6–1000 nIU/ml for insulin, 0.1–100 ng/ml for C-peptide, 0.375–24 ng/ml for adiponectin, 0.02–60 mol/l for TAC, 6.25–350 U/ml for SOD, 40 to 800 U/ml for GPx, 12.50–800 pg/ml for IL-6.
Analysis of gene expression
Total RNA was extracted from liver tissue and adipose tissue using the method described by Chomczynski and Sacchi 1987 and Chomczynski 1993. Total RNA was extracted from 50 mg of each hepatic and adipocyte tissue samples using TRIzol total RNA extraction reagent following the methodology of the TRIzol kit. The resultant cDNA was preserved at −20 °C until used. Semi-quantitative polymerase chain reaction (PCR) used specific primers for the studied genes tested the expression of certain genes participating in the energy metabolism. The tested genes were peroxisome proliferator-activated receptor (PPAR) and insulin receptor substrate-1 (IRS-1), and their housekeeping gene was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Polymerase chain reaction (PCR) was applied using the Thermo Scientific Dream Taq Green PCR Master Mix (2X) as described by Park et al. 2017 with a modification of the annealing temperatures that corresponds to each primer. As a reference (housekeeping gene), expression of GAPDH mRNA was detected using specific primers. Relative quantification of expressed genes calculated as described by Derveaux et al. 2010, as follows: R = 2–ΔΔCt. The primer sequences are as follows:
Oligonucleotide primers sequences:
IRS-1: Forward sequence: 5′ AGAGTGGTGGAGTTGAGTTG3′
Reverse sequence: 5′ GGTGTAACAGAAGCAGAAGC3′
PPAR γ: Forward sequence: 5′GCGGAGATCTCCAGTGATATC 3′
Reverse sequence: 5′TCAGCGACTGGGACTTTTCT3′
GAPDH: Forward sequence: 5′CCATGTTCGTCATGGGTGTGAACCA3′
Reverse sequence: 5′GCCAGTAGAGGCAGGGATGATGTTC3′
Comet assay
Pancreatic β-cell DNA oxidative damage was detected by using comet assay technique following the method described by Tice et al. 2000.
The sample were taken on ice and thawed at 37°C, and the pancreas was washed with PBS containing 20Mm EDTA (Ca+2 and Mg+2 free), and then weighted. For each 200mg of pancreas, 1-ml PBS containing 20Mm EDTA (Ca+2 and Mg+2 free) was added; the tissue was minced using small dissecting scissors into very small pieces, and let stand for 5 min and then the cell suspension was recovered. Counting cells was performed, and then samples were centrifuged at 2800×g for 15 min and suspend in PBS to reach a concentration of 1 x105 cells/ml; 10 μl of cell in PBS were embedded in 95μl of 0.75% low melting point agarose (Gibco-BRL). The mixture (cell/agarose) was added to a fully frosted microscope slide coated with a layer of 300 μl of normal melting agarose (1%) (Gibco-BRL) and spread using coverslips. Then gel was left to solidify at 4°C for 30 min, and then the slides were immersed in the lysis solution for 1 h. Then the slides were immersed in alkaline electrophoresis buffer for 20 min to allow the unwinding and undergo electrophoresis for 15 min at 25 volts and 300 mA. Then, the slides were flooded with 500-μl Tris-HCl 0.4 m, pH 7.5, for 3 times each for 5 min. Then slides were rinsed with distilled water, the slides were flooded with 500-μl absolute ethanol, and the slides were stored in dark humid box until visualization. Each slide was attained with 50-μl ethidium bromide (20 μg/ml) and then visualized under epifluorescent microscope. Scoring of the slide was done according to Collins et al. 1995, using Leica epifluorescent microscope.
Histological examination
Pancreas and liver samples were collected and preserved in 10% formalin solution for histological examination. The specimens of the pancreas and liver from each group were embedded in paraffin wax, and histological section of 5 μm was taken and stained with hematoxylin and eosin (H &E stain) as described by Drury and Wallington 1980. The stained tissue sections were examined under bright-field microscopy (Olympus Optical, Japan) at ×400 magnification. All histological procedures were conducted at the Veterinary Medical Laboratory-Cairo University, Egypt. It must be noted that this histological examination was conducted in a blinded form.
Quantitative scoring of pancreatic and liver lesions was calculated according to Gibson-Corley et al. (2013) with some modification. Briefly, histopathological lesions were scored through determination of the percentage of the lesions in five randomly examined high power (×400) microscopic fields per animal
Statistical analysis
Statistical analysis of results was done using analytical software named SPSS statistics 17.0, Chicago, USA. Values were expressed as means ± S.D. Quantitative differences between values were statistically analyzed by one-way ANOVA, and p values <0.05 were considered to be significant according to Levesque 2007.
Results
Nanoparticles characterization
ZnONPs were successfully synthesized by the co-precipitation method using zinc sulfate and sodium hydroxide as precursors. The TEM image of ZnONPs is depicted in (Fig. 1b). It is observed that ZnONPs have a spherical structure with a mean diameter ranging from 17.0±2.67 nm (Fig. 1a). The UV–Vis spectrum of the prepared ZnONPs is shown in (Fig. 2d). It’s clear that ZnO NPs are semi-conductors with a wide bandgap (3.3eV) that makes it a suitable absorber of UV radiation and exhibit a sharp band at 375 nm. In Fig. 1e, it was shown that the XRD pattern of the as-prepared ZnONPs is very similar to the bulk ZnO, which can be indexed as the hexagonal wurtzite structure ZnO (a = 3.249 Å, c = 5.206 Å) and diffraction data in accordance with JCPDS card for ZnO (JCPDS 36-1451). No other peaks were found which implies that pure ZnO nanostructured were prepared Mohamed and Attia 2020.
Synthesis of Cr2O3NPs was through simply visible light irradiation of the aqueous solution containing potassium chromate, CTAC, and trisodium citrate (Attia et al. 2020). The characterization TEM images of the products are depicted in (Fig. 1c). The average size of the prepared Cr2O3 nanoparticles is 6.0±0.73 nm. The absorption spectra of the performed particles are shown in Fig. 2a. Cr2O3NPs have two absorption peaks located at 423nm and 576nm, respectively (Fig. 2d).(Figure 2 e reports a typical XRD profile obtained on the green Cr2O3NPs powder. The profile shows a series of intense peaks at the various angular positions of ∼24.45°, 33.70°, 36.42°, 40.07°, 50.23°, 54.53°, and 65.59° (Fig. 1a) which indexed to JCPDS card 74-0326 could be ascribed to reflections from the (012), (104), (110), (006), (024), (116), and (300) reticular planes, respectively (Sone et al. 2016).
SeNPs were synthesized by using ascorbic acid as a biocompatible reducing agent in the presence of CTAC as a stabilizing agent. Figure 1 a shows the morphology of the as-prepared SeNPs. The TEM image shows the spherical shape of Se nanoparticles with an average size of 15.22±1.21 nm. When sodium selenite was allowed to react with ascorbic acid, the selenium was reduced to elemental selenium (Se0). The color change from colorless to orange indicated the occurrence of the reduction reaction to form Se NPs. The UV visible absorption spectrum in (Fig. 2d) could manifest such a change in color of the dispersion of SeNPs more clearly. SeNPs showed maximum absorbance at 247 nm. The XRD diffraction peaks of SeNPs (Fig. 2e) are located at 23.63°, 30.04°, 41.23°, 44.46°, 45.71°, 52.01°, and 56.49° that indexed to (100), (101), (110), (102), (111), 200), (201), and (003) lattice planes of hexagonal Se, being in good agreement with the characteristic peaks in the standard card (PDF65-1876) (Emam et al. 2020). The zeta potential data and the mean size of the prepared nanoparticles are shown in Table 1.
Effect of oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture on body weight change and food intake in experimental groups
The present results showed significant weight reduction (p ≤ 0.05) in diabetic control rats (118.37±4.25 g) as compared to healthy control rats (191.50±4.40 g). There was insignificant change between ZnONPs, Cr2O3NPs, and SeNPs treated groups (162.75±5.26, 161.87±5.16, 164.12±3.20 g, respectively). Mixture administered group showed the best weight control effect (185.00±1.71g). Diabetes mellitus caused polyphagia as reflected by increasing food intake in diabetic groups (1291.25±14.93 g/rat) as compared to healthy control group (955.62±10.75g/rat). On the other hand, administration of ZnONPs, Cr2O3NPs, and SeNPs nanoparticles caused significant reduction in food intake (1068.75±16.19, 1045.00±8.45, and 1052.50±11.29g/rat, respectively) as compared to (+ve) control group. Mixture administrated group showed the best improvement and normalization of rat’s body weight and food intake (970.00±8.66 g/rat) as compared to other treated groups.
Effect of oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture on serum glucose, HbA1c %, insulin, c-peptide, HOMA- IR, and adiponectin levels in experimental groups
It is clear from Table 2 that there was a significant increase (p ≤0.05) in serum glucose, HbA1c %, and HOMA-IR, levels as well as a significant decrease (p ≤ 0.05) in serum insulin, c-peptide, and adiponectin levels in the diabetic group as compared to the control group. Furthermore, there were significant increases (p ≤ 0.05) in serum glucose, HbA1c %, and HOMA-IR levels, as well as a significant decrease in serum insulin, C-peptide, and adiponectin levels in the diabetic treated groups as compared to the positive control group. The best ameliorative effect was related to the mixture administered group followed by ZnONPs administered group in glucose homeostasis status. On other hand, for adiponectin level, mixture and SeNPs administered groups showed the best results
Effects of oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture on the liver, kidney functions parameters, and inflammatory biomarkers levels in experimental groups
The data represented in Table 3 showed that there were significant increases (p ≤ 0.05) in serum AST and ALT activities, as well as a marked reduction in albumin levels in the diabetic control group as compared to the healthy control group. Moreover, administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture improved liver function by significantly decreasing serum AST and ALT activities as well as increasing serum albumin levels. The present results proved that the mixture administered group could normalize liver enzymes and showed the best ameliorative effect on AST and ALT activities and albumin level as compared to other diabetic administered groups, followed by SeNPs, Cr2O3NPs, and then ZnONPs administered groups, respectively.
The current results represented that diabetes caused kidney functions disturbance that was manifested by the marked elevation (p ≤0.05) in serum urea, and creatinine levels in the diabetic control group as compared to the healthy control group as showed in the Table 3. Moreover, administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture improved kidney function by reducing levels of serum urea, and creatinine levels. The mixture administered group showed the best improvement in kidney parameters as compared to other nanoparticles administrated groups. Generally, ZnONPs, Cr2O3NPs, and SeNPs administered groups showed equal improvement effects on kidney function parameters.
The current data reported in the Table 4 revealed the anti-inflammatory effect of tested nanoparticles on diabetic rats. The results showed that there was a significant elevation in interleukin-6 (IL-6) and C-reactive protein (CRP) levels in the diabetic control rats compared to the healthy control rats. On the other hand, oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mix to diabetic rats showed a significant reduction in inflammatory markers as compared to the control group. Administration of mixture of nanoparticles succeeded to normalize the levels of IL-6 and CRP.
Effects of oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture treatment on antioxidant/oxidative stress in experimental groups
The results in Table 5 showed that diabetes mellitus caused an antioxidant disturbance that appeared as a significant reduction (p ≤ 0.05) in total antioxidant capacity (TAC) level and erythrocyte glutathione peroxidase (GPx) as well as serum superoxide dismutase (SOD) activities that was accompanied by a significant elevation of serum malondialdehyde (MDA), and on nitric oxide (NO) levels, compared with the healthy control rats. In comparison to the diabetic control group, treatment with ZnONPs, Cr2O3NPs, SeNPs, and their mixture could reflect these effects by significantly increasing erythrocyte GPx and serum SOD activities, as well as TAC levels, while significantly lowering serum MDA and NO levels. Generally, the best antioxidant effect was observed in the mixture-treated group followed by SeNPs, Cr2O3NPs, and then ZnONPs treated groups, respectively.
Effects of oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture on lipid profile levels in experimental groups
It is clear from the current data that diabetes mellitus significantly affected lipid profile as shown in Table 6, while treatment of diabetic rats with ZnONPs, Cr2O3NPs, SeNPs, and their mixture ameliorates these disturbances by significantly reducing TC, TAGs, LDL-C, VLDL-C levels as well as atherogenic coefficient and risk, with significant elevation in HDL-C level on comparing that with the positive control group. Results showed the best hypolipidemic effect that was observed in mix group that approximately normalizes lipid profile levels.
Effects of oral administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture on adipocyte gamma peroxisome proliferator-activated receptor (PPAR-γ) gene, hepatic insulin receptor substrate-1(IRS-1) gene and DNA damage in pancreatic cells by comet assay in experimental groups
Results in Table 7 showed that diabetes mellitus type 2 caused a disturbance in PPAR-γ and IRS-1 gene transcriptional activities leading to glucose homeostasis disturbance and fatty acid oxidation. In the positive control group, the PPAR-γ and IRS-1 mRNA expression were significantly downregulated (p ≤ 0.05) thus reduced the mitochondrial fatty acid oxidation and insulin resistance as compared to the healthy control group. In the treated groups (G3, G4, G5, and, G6), PPAR-γ and IRS-1 mRNA expression were significantly upregulated (p ≤ 0.05) as compared to the diabetic control group. Concerning the results, the mixture administered group showed the most significant upregulation of PPAR-γ and IRS-1 expression.
The percent of DNA damage in pancreatic tissue was significantly increased (p ≤ 0.05) in diabetic rats as compared to the healthy control rats, as shown in Table 8. This was clear from the increased percent of tailed DNA and decreased percent of untailed DNA. Moreover, diabetes mellitus induced a statistically significant increase (p ≤ 0.05) in the average of tail DNA, tail length, and tail moment. These elevations in the comet assay parameters and DNA damage were alleviated by the administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture. Generally, the best protective effect was observed in the mixture treated group followed by SeNP-, Cr2O3NP-, and ZnONP-treated groups, respectively.
Histological examination for hepatic and pancreatic tissues in experimental groups
Histological examination for pancreatic tissues in experimental groups
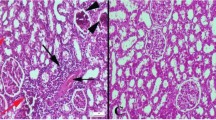
Comparing with the normal histological structure of pancreatic tissues in Fig. 3a showed the normal of islets of Langerhans and exocrine pancreatic acini in the healthy control group (G1). The obtained figures showed that rats fed high fructose diet plus an injection of STZ (G2) revealed severe changes described as marked vacuolation of cells of islets of Langerhan’s with pyknosis of some nuclei Fig. 3b-arrow 1 and necrosis of cells of islets of Langerhans in Fig. 3b-arrow 2 as well as vacuolation of epithelial lining pancreatic acini in Fig. 3b-arrow 3, while pancreatic tissues of diabetic rats that received ZnONPs (G3) showed an improved histological picture as examined sections revealed vacuolation of cells of islets of Langerhan’s with pyknosis of some nuclei (Fig. 3c). On the other hand, diabetic rats that received Cr2O3NPs (G4) showed slight vacuolation of some cells of islets of Langerhans (Fig. 3d). However, examined sections from (G5) diabetic rats that received SeNPs and (G6) diabetic rats that received a mixture of nanoparticles revealed no histopathological changes; examined sections restore the histological architecture of exocrine and endocrine portions which is shown in Fig. 3e and Fig. 3f. The results of the biochemical analysis were in line with microscopic observations of pancreatic tissues.
The microscopic examination of pancreatic cell for the experimental groups. Comparing with the normal histological structure of pancreatic tissues in (a) showed the normal islets of Langerhans and exocrine pancreatic acini in the healthy control group (G1). The rats fed high fructose diet plus an injection of STZ (G2) revealed severe changes described as marked vacuolation (3b-arrow (1)) and necrosis of cells of islets of Langerhans in (3b-arrow (2)) as well as vacuolation of epithelial lining pancreatic acini in (3B arrow (3)). While pancreatic tissues of diabetic rats that received ZnONPs (G3) showed in picture (c), Cr2O3NPs (G4) showed in picture (d), SeNPs (G5) showed in picture (e), and mixture of nanoparticles (G6) showed in picture (f)
Histological examination of liver tissue in experimental groups
Figure 4 showed that, high fructose diet plus injection of STZ, group 2 revealed severe histopathological alterations confined as congestion of central vein and hepatic sinusoids in Fig. 4b1-arrow 1, activation of Kupffer cells in Fig. 4-b1-arrow 2, and cytoplasmic vacuolization of hepatocytes in Fig. 4-b2 comparing with normal histological structure of hepatic parenchyma from normal central vein and hepatocytes arranged in hepatic cords that is shown in the healthy control group (G1) Fig. 4a. While the liver of diabetic rats that received ZnONPs (group 3) revealed slight activation of Kupffer cells (Fig. 4c-arrow 1) as well as slight congestion of central vein (Fig. 4c-arrow 2), on the other hand, diabetic rats that received Cr2O3NPs group (4) revealed slight activation of Kupffer cells (Fig. 4d). Moreover, liver of diabetic rats that received SeNPs group (5) revealed slight Kupffer cells activation in few examined sections (Fig. 4e). Meanwhile, the best treatment effect observed in group (6) diabetic rats received mixture of nanoparticles (ZnONPs + Cr2O3NPs +SeNPs) that shows no histopathological alterations. Note normal hepatic lobule from normal central vein and normal hepatocytes in liver of this group (Fig. 4f). The results of biochemical analysis were in line with microscopic observations of the liver tissues. Effect of nanoparticles treatments on pancreatic cell DNA in type2 diabetic rats as detected by comet assay is shown in Fig.5a–f.
The histological examination of hepatic tissues for the experimental groups. The normal histological structure of hepatic parenchyma from normal central vein (a) and hepatocytes arranged in hepatic cords that shown in the healthy control group (G1). The high fructose diet plus injection of STZ; group (G2) revealed severe histopathological alterations confined as congestion of central vein in (B1-arrow 1), activation of Kupffer cells in (b1-arrow (2)), cytoplasmic vacuolization of hepatocytes (4-b2) comparing with the liver of diabetic rats that received ZnONPs (group 3) which revealed slight activation of Kupffer cells (c-arrow (1)) as well as slight congestion of central vein (c-arrow (2)). On the other hand, diabetic rats that received Cr2O3NPs, group (4) revealed slight activation of Kupffer cells (d). Moreover, liver of diabetic rats that received SeNPs group (5) revealed slight Kupffer cells activation in few examined sections (e). Meanwhile, the best treatment effect observed in group (6) diabetic rats that received mixture of nanoparticles (ZnONPs + Cr2O3NPs +SeNPs) showing no histopathological alterations (f)
Effect of nanoparticles treatments on pancreatic cell DNA in type2 diabetic rats as detected by comet assay. Normal pancreatic cell (a), pancreatic cells from diabetic rats group (b), pancreatic cell from diabetic group treated with ZnONPs (c), pancreatic cell from diabetic group treated with Cr2O3NPs (d), pancreatic cell from diabetic group treated with SeNPs (e), and pancreatic cell from diabetic group treated with Mixture of NPs (f)
Histopathological lesion scores of the pancreas and liver in experimental groups
Table 9 showed the lesion score of the pancreas and liver in experimental groups and the results of histological examination and determination of lesion score go hand in hand with the biochemical parameters
Discussion
The induction of insulin resistance (IR) through fructose feeding in animals has been employed previously. Fructose has been supplied ad libitum either in drinking water or with diets with a concentration of 10–15% for a short or longer period to induce IR or T2DM, respectively, in experimental animals. Unfortunately, the induction of IR, as well as T2DM only by fructose feeding, requires several weeks, which increases the cost of study. Additionally, it has been reported that only fructose feeding for a long period can lead to nutritional tolerance without developing classical signs and symptoms of IR and impaired glucose tolerance (Elankani et al. 2020). Hence, the present study employed the combination of fructose-feeding for a shorter period and a lower dose of STZ injection for inducing all major pathogeneses of T2DM in rats. Current results demonstrated that T2DM caused significant hyperglycemia accompanied with increase in serum, HA1c %, and HOMA-IR and a significant decrease in serum insulin, c-peptide, and adiponectin levels. It can be argued that low C-peptide is merely a marker of β-cell function and low levels indicate β-cell failure. Moreover, hypoadiponectinemia has been shown to precede a decrease in insulin sensitivity as well as predict progression from normoglycemia to prediabetes. The association of higher adiponectin levels with better glycemic control suggests that therapeutic modalities that increase adiponectin levels may be valuable targets for management of T2DM (Abdella and Mojiminiyi 2018). These previously mentioned investigations made us head to use elements as zinc, chromium, and selenium in their nano-form to ameliorate and cure these diabetic complications. On the other hand, the present study is the first study using the mixture of these nanoparticles in treatment of diabetic rats. We adopted that chemical and physical properties of nanoparticles such as size, shape, and surface charge determine their absorption and biodistribution. Absorption, cellular uptake, and clearance of nanoparticles may differ from their larger-sized counterparts. Several researchers have demonstrated enhanced bioavailability of nanoparticles compared with their poor water soluble salts (Nazarizadeh and Asri-Rezaie 2016; De et al. 2008). Thus, the present study evaluated the possible therapeutic effect of single or combined administration of nanoparticles of zinc oxide nanoparticles (ZnONPs), chromium oxide nanoparticles (Cr2O3NPs), and selenium nanoparticles (SeNPs), in fructose/streptozotocin diabetic rat model as a way of enhancing their bioavailability and thereby resulting in antidiabetic effects. Particle solubility, especially in the case of nanoparticles, plays an important role in bioavailability, and hence, the extent of their uptake in the stomach and intestine would be crucial. The uptake of the particulate fraction of nanoparticles from the intestine would then result in slow and continuous release of zinc ions, acting as a depot. Moreover, because of their ultra-small size, they can easily cross through biological membranes and therefore can be highly absorbed by the digestive system. Such characteristics have been eventuated in the utilization of nanoparticles via oral root (Nazarizadeh and Asri-Rezaie2016).
Present results showed a great reduction in blood glucose level in diabetic groups treated with mixture of nanoparticles, ZnONPs, Cr2O3NPs and SeNPs, respectively. This showed a great antidiabetic activity of those nanoparticles. However, ZnONPs induce more reduction than Cr2O3NPs and SeNPs, as zinc has been elucidated to be a potent metal that improves glucose utilization and metabolism through its potent influence on enhancement of hepatic glycogenesis through actions on the insulin signaling pathway (Nazarizadeh and Asri-Rezaie 2016). Current experiments revealed that ZnONPs, Cr2O3NPs, and SeNPs could increase serum insulin level in diabetic groups treated with nanoparticles if compared with diabetic groups, but the best result appear in diabetic group treated with mixture. It appeared that ZnONPs also induced more insulin secretion if compared to the effect of Cr2O3NPs and SeNPs. There are few studies that have investigated the therapeutic effect of ZnONPs on insulin levels or secretion. However, others have demonstrated that zinc could enhance the glucose stimulated insulin secretion from rat isolated pancreatic islets (Richards-Williams et al. 2008).
Interestingly, ZnONPs did not possess the risk of hypoglycemia in living organisms so it can act as insulin secretagogues (Umrani and Paknikar 2014). ZnONPs have several potential mechanisms may be the result of improved glucose tolerance. Firstly, treatment ZnONPs could lead to suppression of intestinal α-glucosidase enzyme and thereby decrease glucose absorption. Secondly, glucose uptake increased in liver and its subsequent storage (glycogenesis) might be lowered blood glucose levels. Thirdly, increased glycolysis by ZnONPs could lead to an increase in glucose disposal. Also, the antidiabetic effects of ZnONPs may be referring to that zinc is directly involved in the overall metabolism of proteins, carbohydrates, and lipids. Zinc is a cofactor of the key enzymes in the case of glucose metabolism. It is a fructose 1-6 diphosphate aldolase activator and a fructose 1-6 diphosphatase inhibitor. Treatment with ZnONPs shows inhibitory effects on glycogenolysis and gluconeogenesis, pathways that are active during the fasted state (Abd El-Rahman et al. 2016). Zinc tends to have insulin-like effects by signaling insulin transduction. Additionally, Zn has a proliferative and protective effect on the pancreatic islets and plays an important role in insulin synthesis, storage, and secretion. Zn increased insulin mRNA and insulin receptors genes expression in hepatic tissues of ZnONPs-receiving diabetic rats. ZnONPs treatment may cause the regeneration of the pancreatic cells and thus increase the level of insulin secretion. These effects were further strengthened by our histological findings as ZnONPs regenerated the islets of Langerhans and mainly restored the normal β cells (Avila et al. 2013)
Similarly, Cr2O3NPs could also increase the insulin level by conjugate with the insulin receptor and induce phosphorylation of insulin receptor substrates (IRS-1 and IRS-2) which play a vital role in transmitting signals from insulin to intracellular metabolic pathways, including phosphatidylinositol 3-kinase (PI3K). PI3K has a principal role in the function of insulin, through the activation of protein kinase B (PK-B). PI3K and PK-B have a vital role in the translocation of (GLUT4). Cr2O3NPs administration to diabetic rats increases the expression of insulin signaling cascade biomarkers in the liver through increasing IRS-1 and PI3K levels. Cr2O3NPs enhanced the tyrosine phosphorylation of insulin receptors in addition to enhancing the insulin receptor tyrosine kinase activity in the membranes fraction of the chromium-treated cells, as demonstrated by increased levels of IRS-1 tyrosine phosphorylation, chromium could upregulate insulin-stimulated insulin signal transduction by influencing molecules downstream of the IR (El-Megharbel 2015). Administration of Cr2O3NPs to diabetic rats prevented the significant elevation in glycosylated hemoglobin, thereby increasing the level of total hemoglobin in diabetic rats (El-Megharbel 2015). This could be due to the improved glycemic control produced by this new complex (mix of nanoparticles). On the other hand, SeNPs have various mechanisms in acting as hypoglycemic agent. Firstly, by reducing oxidative stress and reducing the oxidation of lipoproteins, SeNPs can play a key role in improving diabetes through their antioxidant activity. Secondly, the impacts of SeNPs on blood sugar regulation such as insulin signaling, glycolysis, and pyruvate metabolism. Thirdly, SeNPs enhance glucose uptake in fat cells taken from rats. This effect was caused by increased transport of glucose to the plasma membrane and serine/threonine kinase activation. Fourthly, SeNPs can improve diabetes by participating in GPx1. GPx-1 plays an effective role in protecting pancreatic β-cells against damage that is caused by STZ. SeNPs exert their anti-diabetic effects by protecting the integrity of the β-cells, enhanced insulin secretion. So, the synergic effects of the three metals in mixture make it the best effector on insulin secretion and hypoglycemic effect.
In addition, present results indicated that T2DM caused hepatic and renal dysfunction that was synchronized with dyslipidemia. The increases in the activities of ALT and AST in the serum of diabetic rats may be mainly due to leakage of these enzymes from liver cytosol into the bloodstream as a consequence of hepatic injury-associated DM. Hepatic inflammation induced by diabetes mellitus pathogenesis is probably another reason for albumin level decrease in the blood (Rezaei-Kelishadi et al. 2017). Insulin deficiency or insulin resistance may be responsible for dyslipidemia, because insulin has an inhibitory action on HMG-coA reductase, a key rate-limiting enzyme responsible for the metabolism of cholesterol-rich LDL particles (Hussein et al .2014).
Our results show that administration of ZnONPs, Cr2O3NPs, SeNPs, and their mixture improved liver and kidney function toward normal levels, but the best ameliorative effect appears in mixture-treated group, followed by SeNPs treated group. The results of the present study indicated that SeNPs are of beneficial effects on liver functions and restored the activities of ALT and AST toward normal levels as well as the level of albumin. These finding could be explained due to the radical scavenging property of Se and the importance of Se in protecting the integrity and the functions of tissues (Majeed et al. 2018). These results could be due to the role of SeNPs in protecting the integrity and functions of liver tissues besides its radical scavenging activity. The observed renal dysfunction in HFD/STZ rats was reversed by treatment with SeNPs. The hypoglycemic effect of SeNPs may explain the ameliorated renal function in the treated rats. Treatment with SeNPs leads to the recovery and restoration of endothelial dysfunction and vascular disorders through regulating antioxidant enzymes and releasing nitric oxide.
It is well known that diabetic hyperglycemia has a major role in the induction of oxidative stress. Data generated in the present investigation demonstrated augmentation of oxidative stress in diabetic rats with concomitant impairment in the antioxidant defense systems, as observed by a marked elevation in the oxidative stress biomarker (MDA and NO) and reduction in the free-radical neutralizing antioxidants, including SOD, GPx, and TAC. Meanwhile, administration of ZnONPs, Cr2O3NPs, and SeNPs significantly elevated the GPx and SOD activities and significantly reduced MDA and NO levels in diabetic rats. Surprisingly, mixture-treated group showed normalization of GSH and SOD activities as well as NO and MDA levels as compared to control group. Moreover, mixture administered group have a significant increase in TAC than other treated groups. On the other hand, SeNPs treated group has more effective antioxidant activity than ZnONPs and Cr2O3NPs treated groups. This refers to the role of SeNPs in recovery and restoration of endothelial dysfunction and vascular disorders through regulating antioxidant enzymes and releasing nitric oxide (Oztürk et al. 2015). One of the most approved hypotheses of Se antioxidant activity is the potential of SeNPs to keep the glutathione in the reduced form where glutathione has the ability to detoxify the ROS. The activity of SeNPs is achieved by the selenium-containing enzyme GPx which protects cells against ROS (Usrey et al. 2020). Ukperoro et al. 2010 reported that Zn may either increase the biosynthesis of GSH or reduce the oxidative stress contributing to less degradation of GSH or have both effects. This provided a potential mechanism that Zn deficiency increase progress of DM. This provides a potential mechanism for Zn deficiency to improve DM development. In addition, Cr2O3NPs greatly scavenged free radical molecules and thus decreased MDA level as it is the final product of lipid peroxidation and increased the enzymatic capacities of SOD and GPx and thus increasing TAC level, improving liver function tests, and thus enhancing the conversion of blood glucose into glycogen and thus decreasing level of blood glucose which reflects the solution for diabetes mellitus complications (El-Megharbel 2015). Supplementation of Cr2O3NPs to diabetic rats significantly modulated the alterations in oxidative stress marker (NO) and antioxidant biomarkers levels in serum. Cr2O3NPs administration inhibits NO production and iNOS protein expressions. Also, Cr can attenuate diabetic oxidative stress and improve antioxidant capacity in cell culture and experimental animals (Hua et al. 2012). The modulating effect of Cr2O3NPs on antioxidant biomarkers and oxidative stress and may be related to its glucoregulatory effect as documented in the current study. Insulin deficiency or insulin resistance occurred in T2DM may be responsible for dyslipidemia, because insulin has an inhibitory action on HMG-coA reductase, a key rate-limiting enzyme responsible for the metabolism of cholesterol-rich LDL particles (Hussein et al. 2014). The results showed the hypolipidemic effects of ZnONPs, Cr2O3NPs, and SeNPs treated groups in approximately equal manner. Mixture-treated group showed the best hypolipidemic effect that approximately normalizes lipid profile levels well as atherogenic coefficient and risk. The beneficial effect of ZnONPs on lipid profile levels suggests possible insulin-mimetic effects in adipocytes. It has recently been reported that zinc ions inhibit adipocyte lipolysis and non-esterified fatty acids release through inhibition of hormone-sensitive lipase. Similar insulin mimetic mechanisms may be operative in the case of ZnONPs (Umrani and Paknikar 2014). Chromium nanoparticles was able to mitigate diabetic abnormalities through decreasing serum glucose, triacylglycerols, total cholesterol, and IL-6 and increasing adiponectin, HDL, insulin, and GSH concentrations as well as increased PPAR-γ gene expression in adipose tissue, which may exert a potent insulin-sensitizing effect and may improve insulin resistance in a rat model of type 2 diabetes mellitus (Abou zaid et al. 2015). After the oral administration of SeNPs, a remarkable decrease in serum levels of TAGs, TC, and LDL-C was observed linked with a significant increase in HDL-c levels compared to the untreated diabetic rats. The decrease TAGs and TC levels in the current study may be due to the action of HDL-C that could raise the efflux of TG and TC to liver tissue for catabolism.
In present work, hyperglycemia as a significant stressor caused chronic inflammation. Hyperglycemia induces IL-6 production from endothelium and macrophages, which might worsen insulin liberation and signaling cascades. Elevated levels of IL-6 promote insulin resistance in skeletal muscle and endothelial dysfunction, as well as liberation of CRP from the liver (Ukperoro et al. 2010). The current study revealed that oral administration of ZnONPs Cr2O3NPs, SeNPs, and their mixture to diabetic rats showed a significant reduction in inflammatory markers as compared to the control group. The best anti-inflammatory effect was reported in mixture group that success in normalize the levels of IL-6 and CRP in diabetic rats. ZnONPs negatively regulated gene expression of inflammatory cytokines such IL-1 and IL-6, which are known to generate ROS. ZnONPs have also been proposed to interact with cell membranes to stabilize them against oxidative damages Ukperoro et al. 2010. Meanwhile, Cr2O3NPs inhibit the secretion of pro-inflammatory cytokines (i.e., IL-6) and reduce oxidative stress in monocytes exposed to high glucose and standard oxidants like H2O2. Furthermore, chromium nanoparticles were able to mitigate diabetic abnormalities through decreasing serum glucose, and IL-6 and increasing adiponectin level (Abou Zaid et al. 2015). Several studies have shown that SeNPs downregulate mRNA expression of pro-inflammatory cytokines including inducible NO synthase (iNOS), interleukin IL-1, and TNF-α thus reducing inflammation (Shi et al. 2011; Wang et al. 2016). Selenium is known as a fundamental component of selenoprotein, an essential enzyme in the body that protects against oxidative stress and inflammation (Majeed et al. 2018).
A significant decrease in gene expression of peroxisome proliferator activated receptor gamma (PPAR-γ), which is evaluated by relative quantification real-time quantitative PCR that was observed in diabetic rats. These results go in hand with those reported by Rotter et al. 2002 who demonstrated that adipocytes secrete various proteins, called adipocytokines, which influence insulin sensitivity profoundly thereby providing possible molecular link between increased adiposity and reduced PPAR-γ. IRS-1 appears to play a central role in the insulin-stimulated signal transduction pathway and links the insulin receptor to its final biological actions via a series of intermediate effectors (Afifi et al. 2020). In the present study, decreases of IRS-1 expression in the liver were observed in diabetic rats as in other study reported by Sahin et al. 2013. Our results pointed out that mRNA expression levels of PPAR-γ and IRS-1 gene appeared to increase in ZnONPs, Cr2O3NPs, and SeNPs treated groups if compared with the diabetic untreated group, and the best result found in mixture treated group. The present study clearly showed that supplementing Cr2O3NPs or SeNPs exert antioxidant; anti-hyperlipidemic, anti-inflammatory, and anti-hyperglycaemic effects, and increases the level of insulin in diabetic rats. These changes may be related to the elevation of the levels of PPAR-γ and IRS-1 in the liver, kidney, and muscle tissues.
By using comet assay, current study revealed that diabetes cause DNA damage. This result is similar to that obtained from the study of Sardas et al. 2001.The current data revealed that the best protective effect was observed in mix-treated group followed by SeNPs, Cr2O3NPs, and ZnONPs treated groups, respectively. The possible causes for selenium to have more protective effect on DNA rather than other NPs may be due to the antioxidant property of a compound which could play a significant role in decreasing the nuclear injury caused by diabetes. SeNPs have antidiabetic potency, repression of oxidative stress, potentiating of the antioxidant defense system, and inhibition of pancreatic inflammation. So, selenium plays a protective role against type 2 diabetes. The protective roles of selenium in mammalian cell are due to its function in the active site of many antioxidant enzymes, such as thioredoxin reductase, and glutathione. Dietary selenium may cause an increase in glutathione peroxidase, which has the ability to detoxify ROS that can interfere with insulin signaling and result in regulation of glucose levels and prevention of diabetes (Abd El-Rahim et al. 2017). The measurement of the plasma levels of these nanomaterials in rats under these experimental conditions and COVID-19 pandemic were the limitations of this study.
Conclusion
Based on the present study, it could be concluded that ZnONPs, Cr2O3NPs, and SeNPs have significant antidiabetic effect in rats. They also showed good hypolipidemic, antioxidant, and anti-inflammatory properties as well as DNA protecting activity and genotoxicity ameliorative effects. The strongest ameliorative and antidiabetic effects have been shown by the mixture of these nanoparticles, which can be due to the synergistic impact of many mechanisms of each compound that may have a different mechanism of action. For the assessment of therapeutic efficacy, the mechanism of action of the observed anti-diabetic effect of the mixture is warranted and is considered a healthy supplementary therapy for T2DM. It can also help to reduce diabetic complications and can act as a good alternative to antidiabetic drugs. Overall, the current work indicates that a mixture of ZnONPs, Cr2O3NPs, and SeNPs represents a new formulation for achieving an antidiabetic activity.
References
Abd El-Rahim HA, Abd-Elmoneim OM, Hafiz NA (2017) Assessment of antigenotoxic effect of nanoselenium and metformin on diabetic rats. Jordan Journal of Biological Sciences 10(3):159–165
Abd El-Rahman NS, Reda SM, Al Ghannam MS (2016) Synthesis and characterization of nano-doped zinc oxide and its application as protective oxidative changes in the retina of diabetic rats. Journal of Diabetes & Metabolism 7(7):1–6
Abdella AN, Mojiminiyi AO (2018) Clinical applications of adiponectin measurements in type 2 diabetes mellitus. Screening Diagnosis and Marker of Diabetes Control 2018(6):1–6
Abdourahman A, Edwards JG (2008) Chromium supplementation improves glucose tolerance in diabetic Goto-Kakizaki rats. IUBMB Life 60(8):541–548
Abou Zaid RA, El-sonbaty MS, Afifi FWM (2015) The biochemical effect of chromium nanoparticles administration on adiponectin secretion, oxidative stress and metabolic disordered in streptozotocin induced diabetic rats. Benha Veterinary Medical Journal 28(1):266–275
Afifi M, Alkaladi A, Abomughaid M, Abdelazim AM (2020) Nanocurcumin improved glucose metabolism in streptozotocin-induced diabetic rats: a comparison study with Gliclazide. Environ Sci Pollut Res 27(7):271–277
Ahmed HH, Abd El-Maksoud MD, AbdelMoneim AE, Aglan HA (2017) Pre-clinical study for the antidiabetic potential of selenium nanoparticles. Biol Trace Elem Res 177(2):267–280
Allain C, Poon L, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20(4):470–475
Anderson RA (1998) Chromium, glucose intolerance and diabetes. J Am Coll Nutr 17(6):548–555
Anderson RA, Polansky MM, Bryden NA, Canary JJ (1991) Supplemental-chromium effects on glucose, insulin, glucagon, and urinary chromium losses in subjects consuming controlled low-chromium diets. Am J Clin Nutr 54(5):909–916
Avila DL, Araujo GR, Silva M, Miranda PHA, Diniz MF, Pedrosa ML, Silva ME, Lima WG, Costaa DC (2013) Vildagliptin Ameliorates Oxidative Stress and Pancreatic Beta Cell Destruction in Type 1 Diabetic Rats. Archives of Medical Research 44(3):194–202
Attia AY, Attia YS, Essa R, Mohamed S (2020) Photosynthesis of chromium oxide/reduced graphene oxide nanocomposites and its application in water desalination and purification. nanoscience & nanotechnology-Asia 10 (5). https://doi.org/10.2174/2210681209666190627155956
Berenjian A, Sharifi DS, Abdollah MSA, Ghazanfari A (2018) Effect of chromium nanoparticles on physiological stress induced by exogenous dexamethasone in Japanese quails. Biol Trace Elem Res 184:474–481
Brehm A, Pfeiler G, Pacini G, Vierhapper H, Roden M (2004) Relationship between serum lipoprotein ratios and insulin resistance in obesity. Clin Chem 50:2316–2322
Bulau P, Zakrzewicz D, Kitowska K, Leiper J, Gunther A, Grimminger F, Eickelberg O (2007) Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol 292:18–24
Butler AR (1975) The Jaffe reaction identification of the colored species. Clin Chim Acta 59:227–232
Chausmer AB (1998) Zinc, insulin and diabetes. J Am Coll Nutr 17(2):109–115
Chen C, Yu H, Zhao J, Li B, Qu L, Liu S, Zhang P, Chai Z (2006) The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ Health Perspect 114(2):297–301
Chen WY, Chen CJ, Liu CH, Mao FC (2009) Chromium supplementation enhances insulin signalling in skeletal muscle of obese KK/HlJ diabetic mice. Diabetes Obes Metab 11(4):293–303
Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 15:532–537
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Collins S, Glenn SM, Gibson DJ (1995) Experimental Analysis of Intermediate Disturbance and Initial Floristic Composition: Decoupling Cause and Effect. Ecology 76(2):486–492
De M, Ghosh PS, Rotello VM (2008) Applications of nanoparticles in biology. Adv Mater 20(22):4225–4241
Derveaux S, Jo V, Jan H (2010) How to do successful gene expression analysis using real-time PCR. Methods. 50:227–230
Dobiásová M, Frohlich J (2001) The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoBlipoprotein-depleted plasma (FER(HDL)). Clin Biochem 34(7):583–588
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and measurements of serum albumin with bromocresol green. Clin Chim Acta 31:87–96
Drury RA, Wallington EA (1980) Carletons histology technique, 4th edn. Oxford university press, New York
Elankani G, Reddy D, Kabilan N, Kumar P, Ganesan R, Kumar N, Pitchaiah D (2020) Evaluation of the anti-diabetic activity of the tran vidhai kudineer on fructose and streptozotocin induced diabetic rats. IJPSR. 11(5):2282–2292
El-Megharbel SM (2015) Synthesis, characterization and antidiabetic activity of chromium (III) metformin complex. Journal of Microbial & Biochemical Technology 7(2):065–075
Emam M, Keshta TA, Mohamed AMY, Attia AY (2020) Insight on ameliorative role of selenium nanoparticles and niacin in wound healing on adult female albino mice. Curr Chem Biol 14:169–186. https://doi.org/10.2174/2212796814666200818111849
Fassati P, Prencipe L (1982) Measurement of serum TG calorimetrically with an enzyme that produce H2O2. Clin Chem 28:2077–2080
Fawcett JK, Scott JE (1960) A rapid and precise of method for the determination of urea. J Clin Pathol 13:156–160
Friedewald WT, Robert IL, Donald SF (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(16):499–502
Gandhi GR, Sasikumar P (2012) Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed 2:281–286
Ghosh D, Bhattacharya B, Mukherjee B, Manna B, Sinha M, Chowdhury J, Chowdhury S (2002) Role of chromium supplementation in Indians with type 2 diabetes mellitus. J Nutr Biochem 13(11):690–697
Gibson-Corley KN, Olivier AK, Meyerholz DK (2013) Principles for Valid histopathologic scoring in research. Vet Pathol 50:1007–1015
Hirano T (1998) c. In: Thomson AE (ed) The cytokine. Handbook 3rd edition. Academic Press, San Diego, pp 197–227
Hua Y, Clark S, Ren J, Sreejayan N (2012) Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem 23(4):313–319
Hussein SA, YA EL-S, El-Dawy K, Hind AB (2014) Evaluation of zinc oxide nanoparticles for insulin, insulin receptors and insulin receptors substrates gene expression in streptozotocin-induced diabetic rats. Benha Veterinary Medical Journal 27(1):166–174
Jansen J, Karges W, Rink L (2009) Zinc and diabetes – clinical links and molecular mechanisms. J Nutr Biochem 20(6):399–417
Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P (2012) Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 4(1):13
Kumar C, Kumar R, Nehar S (2014) Induction of type 2 diabetes by high –fructose diet and low dose of intraperioneal injection of STZ in albion rats. Int J Pharm Res Sch 3(1):196–202
Levesque R (2007) SPSSS programming and data management: a guide for SPSS and SAS user. 3rd edition, United States of America.
Lopes-Virella MF, Stone P, Ellis S, Colwell JA (1977) Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem 23:882–884
Majeed W, Zafar M, Bhatti A, john P (2018) Therapeutic potential of selenium nanoparticles. Journal of Nanomedicine & Nanotechnology 9:1.
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Mohamed AMY, Attia AY (2020) The influence of ultrasonic irradiation on catalytic performance of ZnO nanoparticles towards the synthesis of chiral 1-substituted-1H-tetrazole derivatives from α-amino acid ethyl esters. Appl Organomet Chem 34(9):57–58
Mueller AS, Mueller K, Wolf NM, Pallauf J (2009) Selenium and diabetes: an enigma? Free Radic Res 43(11):1029–1059
Nathan DM, Singer DE, Hurxthal K, Goodson JD (1984) The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 310:341–346
Nazarizadeh A, Asri-Rezaie S (2016) Comparative study of antidiabetic activity and oxidative stress induced by zinc oxide nanoparticles and zinc sulfate in diabetic Rats. AAPS PharmSciTech 17(4):834–843
O’Rahilly S, Moller DE (1992) Mutant insulin receptors in syndromes of insulin resistance. Clinical Endocrinology (Oxf) 36:121–132
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Othman SM, Hafez MM, Abdel Moneim EA (2019) The Potential role of zinc oxide nanoparticles in micrornas dysregulation in STZ-induced type2 diabetes in rats. Biol Trace Elem Res 12:1–13
Oztürk Z, Gurpinar T, Vural K, Boyacıoglu S, Korkmaz M, Var A (2015) Effects of selenium on endothelial dysfunction and metabolic profile in low dose streptozotocin induced diabetic rats fed a high fat diet. Biotech Histochem 90:506–515
Park C, Kim J, Lee E, Hur W, Kwon O, Park H, Yoon S (2017) Aronia melanocarpa extract ameliorates hepatic lipid metabolism through PPARγ2 downregulation. PLoS One 12(1):1–22
Reeves PG, Nielsen FH, Fahey JR (1993) AIN-93 Purified diets for laboratory rodents: final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A Rodent Diet. J Nutr 26(123):1939–1951
Reitman S, Frankel S (1957) Colourimetric determination of serum GOT [glutomic oxaloacetic transsaminase] activity. Am J Clin Pathol 28:56–63
Rezaei-Kelishadi M, Ghasemi A, Abdolyosefi NN, Zamani-Doabi S, Ramezani M, Changizi-Ashtiyani S, Rahimi A (2017) Effects of selenium nanoparticles on kidney and liver functional disorders in streptozotocin-induced diabetic rats. J Physiol Pharmacol 21:155–162
Richards-Williams C, Contreras LJ, Berecek HK, Schwiebert ME (2008) Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic Signal 4:393–405
Ronald D (1997) Quantitative determination of low levels of C-Reactive Protein (CRP). Journal of Clinical Ligand Assay:313–315
Rotter V, Nagaev I, Smith U (2002) Interleukin-6 (IL-6) reduces gene and protein expression of IRS-1 and GLUT4 and is overexpressed in human fat cells from insulin-resistant subjects. Diabetes 51:303
Rungby J (2010) Zinc, zinc transporters and diabetes. Diabetologia 53(8):1549–1551
Sahin K, Tuzcu M, Orhan C, Sahin N, Kucuk O, Ozercan HI, Ibrahim HO, Juturu V, Komorowski RJ (2013) Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br J Nutr 110:197–205
Sardas S, Yilmaz M, Oztok U, Caki N, Karakaya AE (2001) Assessment of DNA strands breakage by comet assay in diabetic patients and the role of antioxidant supplementation. Mutat Res 490(2):123–129
Shi L, Xun W, Yue W, Zhang C, Ren Y (2011) Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin Res 96:49–52
Shihabudeen MS, Roy D, James J, Thirumurugan K (2015) Chenodeoxycholic acid, an endogenous FXR ligand alters adipokines and reverses insulin resistance. Molecular and Cellular Endocrinology 414:19–28
Smidt K, Jessen N, Petersen AB, Larsen A, Magnusson N, Jeppesen JB, Stoltenberg M, Culvenor JG, Tsatsanis A, Brock B, Schmitz O, Wogensen L, Bush AI, Rungby J (2009) SLC30A3 Responds to Glucose- and Zinc Variations in ßCells and Is Critical for Insulin Production and In Vivo Glucose-Metabolism During ß-Cell Stress. PLoS ONE 4(3):e5684
Sone BT, Manikandan E, Gurib-Fakim A, Maaza M (2016) Single-phase α-Cr2O3 nanoparticles’ green synthesis using Callistemon viminalis’ red flower extract. Green Chemistry Letters and Reviews 9(2):85–90
Srivastava P, Braganca JM, Kowshik M (2014) In vivo synthesis of selenium nanoparticles by Halococcus salifodinae BK18 and their anti-proliferative properties against HeLa cell line. Biotechnol Prog 30:1480–1487
Steinbrenner H, Sies H (2009) Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 1790(11):1478–1485
Tag-ElDin N (2019) Effects of dietary nano-Zinc and nano-selenium addition on productive and physiological performance of growing rabbits at fattening period. Egyptian Journal of Nutrition and Feeds 22(1):79–89
Thompson KH, Lichter J, LeBel C, Scaife MC, McNeill JH, Orvig C (2009) Vanadium treatment of Type 2 diabetes: a view to the future. J Inorg Biochem 103(4):554–558
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Trachootham D, Lu W, Weiqin L, Marcia AO, Del R, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374
Trinder L (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann Clin Biochem 6:24–27
Ukperoro JU, Offiah N, Idris T, Awogoke D (2010) Antioxidant effect of zinc, selenium and their combination on the liver and kidney of alloxan-induced diabetes in rats. Mediterr J Nutr Metab 3(1):25–30
Umrani DR, Paknikar KM (2014) Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Types 1 and 2 diabetic rats. Nanomedicine 9(1):89–104
Ursini F, Maiorion M, Gregolin CT (1985) the selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 839:62–70
Usrey E, Fatah-Allah NG, Hany M, Mohsen AE, Maher AAA (2020) Biochemical and histopathological studies on the role of selenium nanoparticles and selenium oxide against chemically-induced diabetes in male rats. Research Article 2(2) e scientific:1–10
Vikram A, Jena G, Ramarao P (2011) Insulin-resistance reduces botulinum neurotoxin-type A induced prostatic atrophy and apoptosis in rats. Eur J Pharmacol 650:356–363
Wang ZQ, Cefalu WT (2010) Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Current diabetes reports 10(2):145–151
Wang XL, Yang TB, Wei J, Lei GH, Zeng C (2016) Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta-analysis of observational studies. Nutr J 15(1):48
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
World Health Organization. (2014) "About diabetes. Retrieved 4 April.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RH, ME, and YA conceived, designed research, analyzed data, wrote the manuscript, and reading and approving the manuscript. TK, NH, and JG conducted experiments and contributed with reagents and test methods and analytical tools. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors hereby declare that "Principles of laboratory animal care" (NIH publication No. 85-23, revised 1985) were followed, as well as specific national laws where applicable. All animal experiments were performed under protocol approved by the Local Institutional Animal Ethics Committee of Ain Shams University.
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interest.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassan, R.M., Elsayed, M., Kholief, T.E. et al. Mitigating effect of single or combined administration of nanoparticles of zinc oxide, chromium oxide, and selenium on genotoxicity and metabolic insult in fructose/streptozotocin diabetic rat model. Environ Sci Pollut Res 28, 48517–48534 (2021). https://doi.org/10.1007/s11356-021-14089-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14089-w