Abstract
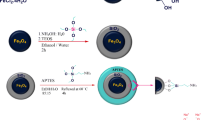
The magnetic Fe3O4 was synthesized by using a one-step solvothermal method. Then, anhydrous ethanol as a solvent, tetramethyl ammonium hydroxide (TMAOH) as an auxiliary agent, tetraethyl orthosilicate (TEOS) as a silicon source, and (3-aminopropyl) triethoxysilane (APTES) as amino source were used to prepare Fe3O4@mSiO2-NH2 by using the sol-gel method. Uniform design U14*(145) and the response surface method (RSM) were used to optimize the synthesis ratio. According to the results of TEM, SEM, N2 adsorption–desorption test, VSM, and XRD, it found that the best coating effect obtained when the relative molar ratio of TMAOH:TEOS:APTES:Fe3O4 was 5:4:6:0.45. The results of EDS and elemental analysis confirmed the success of amino group coating; VSM magnetization after surface modification was 32 emu/g; BET results show that specific surface area is 236 m2/g, size 5 nm, and the pore volume is 0.126 cm3/g. The removal of Cu2+, Zn2+, and Pb2+ by Fe3O4@mSiO2-NH2 was studied at the optimal initial pH value 6 of the adsorption test system. The isothermal adsorption results show that the Langmuir model and Redlich–Peterson model are more suitable than the Freundlich model to describe the adsorption behavior, and Cu2+, Zn2+, and Pb2+ adsorption is mainly single molecular layer. The maximum adsorption capacity qm of the Langmuir model for Cu2+, Zn2+, and Pb2+ removal was 48.04 mg/g, 41.31 mg/g, and 62.17 mg/g, respectively. The adsorption kinetic rates of Cu2+, Zn2+, and Pb2+ on Fe3O4@mSiO2-NH2 relatively more suitable for pseudo-second-order kinetic model, i.e., R2, were ranged between 0.995 and 0.999, and the suitable reaction time was 60 min. These results proved that Fe3O4@m-SiO2-NH2 prepared by using this method is easy to synthesize, has easy recovery, is ecofriendly, and can be potential adsorbent for Cu2+, Zn2+, and Pb2+ removal.














Similar content being viewed by others
Data availability
Not applicable.
References
Ayawei N, Ekubo AT, Wankasi D, Dikio ED (2015) Adsorption of congo red by Ni/Al-CO3: equilibrium, thermodynamic and kinetic studies. Orient J Chem 31:1307–1318. https://doi.org/10.13005/ojc/310307
Belhachemi M, Belalaa Z, Lahcenea D, Addounb F (2009) Adsorption of phenol and dye from aqueous solution using chemically modified date pits activated carbons. Desalin Water Treat 7:182–190. https://doi.org/10.5004/dwt.2009.729
Brouers F, Al-Musawi TJ (2015) On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J Mol Liq 212:46–51. https://doi.org/10.1016/j.molliq.2015.08.054
Chabicovsky M, Klepal W, Dallinger R (2004) Mechanisms of cadmium toxicity in terrestrial pulmonates: programmed cell death and metallothionein overload. Environ Toxicol Chem 23:648–655. https://doi.org/10.1897/02-617
Chen D, Awut T, Liu B, Ma Y, Wang T, Nurulla I (2016) Functionalized magnetic Fe3O4 nanoparticles for removal of heavy metal ions from aqueous solutions. E-Polymers 16:313–322. https://doi.org/10.1515/epoly-2016-0043
Fang KT, Lin DKJ, Winker P, Zhang Y (2000) Uniform design: theory and application. Technometrics 42:237–248. https://doi.org/10.1080/00401706.2000.10486045
Geetha KSGK (2013) Removal of heavy metals and dyes using low cost adsorbents from aqueous medium-, a review. IOSR J Environ Sci Toxicol Food Technol 4:56–68. https://doi.org/10.9790/2402-0435668
Ghasemi N, Ghasemi M, Moazeni S, Ghasemi P, Alharbi NS, Gupta VK, Agarwal S, Burakova IV, Tkachev AG (2018) Zn (II) removal by amino-functionalized magnetic nanoparticles: kinetics, isotherm, and thermodynamic aspects of adsorption. J Ind Eng Chem 62:302–310. https://doi.org/10.1016/j.jiec.2018.01.008
Huang X, Yang J, Wang J, Bi J, Xie C, Hao H (2018) Design and synthesis of core–shell Fe3O4@PTMT composite magnetic microspheres for adsorption of heavy metals from high salinity wastewater. Chemosphere 206:513–521. https://doi.org/10.1016/j.chemosphere.2018.04.184
Järup L (2002) Cadmium overload and toxicity. Nephrol Dial Transplant 17:35–39. https://doi.org/10.1093/ndt/17.suppl_2.35
Jin S, Park BC, Ham WS, Pan L, Kim YK (2017) Effect of the magnetic core size of amino-functionalized Fe3O4-mesoporous SiO2 core-shell nanoparticles on the removal of heavy metal ions. Colloids Surfaces A Physicochem Eng Asp 531:133–140. https://doi.org/10.1016/j.colsurfa.2017.07.086
Jin W, Ma J, Ma H, Li X, Wang Y (2018) Hydrothermal synthesis of core-shell ZSM-5/SAPO-34 composite zeolites and catalytic performance in methanol-to-aromatics reaction. J Solid State Chem 267:6–12. https://doi.org/10.1016/j.jssc.2018.08.004
Kleijnen JPC, Den Hertog D, Angün E (2004) Response surface methodology’s steepest ascent and step size revisited. Eur J Oper Res 159:121–131. https://doi.org/10.1016/S0377-2217(03)00414-4
Kwon JS, Yun ST, Lee JH, Kim SO, Jo HY (2010) Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic(III) from aqueous solutions using scoria: kinetics and equilibria of sorption. J Hazard Mater 174:307–313. https://doi.org/10.1016/j.jhazmat.2009.09.052
Lachowicz JI, Delpiano GR, Zanda D, Piludu M, Sanjust E, Monduzzi M, Salis A (2019) Adsorption of Cu2+ and Zn2+ on SBA-15 mesoporous silica functionalized with triethylenetetramine chelating agent. J Environ Chem Eng 7:103205. https://doi.org/10.1016/j.jece.2019.103205
Lakherwal D (2014) Adsorption of heavy metals: a review. Int J Environ Res Dev 4:2249–3131. https://doi.org/10.1007/s11270-007-9401-5
Lee HW, Cho HJ, Yim JH, Kim JM, Jeon JK, Sohn JM, Yoo KS, Kim SS, Park YK (2011) Removal of Cu(II)-ion over amine-functionalized mesoporous silica materials. J Ind Eng Chem 17:504–509. https://doi.org/10.1016/j.jiec.2010.09.022
Li Y, He J, Zhang K, Liu T, Hu Y, Chen X, Wang C, Huang X, Kong L, Liu J (2019) Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Adv 9:397–407. https://doi.org/10.1039/C8RA08638A
Liang YZ, Fang KT, Xu QS (2001) Uniform design and its applications in chemistry and chemical engineering. Chemom Intell Lab Syst 58:43–57. https://doi.org/10.1016/S0169-7439(01)00139-3
Lim AP, Aris AZ (2014) A review on economically adsorbents on heavy metals removal in water and wastewater. Rev Environ Sci Biotechnol 13:163–181. https://doi.org/10.1007/s11157-013-9330-2
Lin L, Thirumavalavan M, Wang Y, Lee J (2010) Effect of preparation conditions on the adsorption of heavy metal ions from aqueous solution by mesoporous silica materials prepared using organic template ( HDTMAB ). 3667–3673
Lin Y, Chen H, Lin K, Chen B, Chiou C (2011) Application of magnetic particles modified with amino groups to adsorb copper ions in aqueous solution. J Environ Sci 23:44–50. https://doi.org/10.1016/S1001-0742(10)60371-3
Liao Q, Zeng L et al (2014) Desalination and water treatment synthesis of functionalized mesoporous silica and its application for Cu (II) removal. https://doi.org/10.1080/19443994.2014.958762
Liu L, Luo X-B, Ding L, Luo S-L (2019) Application of nanotechnology in the removal of heavy metal from water. Elsevier Inc.
Maheshwari U, Mathesan B, Gupta S (2015) Efficient adsorbent for simultaneous removal of Cu(II), Zn(II) and Cr(VI): kinetic, thermodynamics and mass transfer mechanism. Process Saf Environ Prot 98:198–210. https://doi.org/10.1016/j.psep.2015.07.010
Meng C, Zhikun W, Qiang L, Chunling L, Shuangqing S, Songqing H (2018a) Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J Hazard Mater 341:198–206. https://doi.org/10.1016/j.jhazmat.2017.07.062
Meng C, Zhikun W, Qiang L, Chunling L, Shuangqing S, Songqing H (2018b) Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J Hazard Mater 341:198–206. https://doi.org/10.1016/j.jhazmat.2017.07.062
Mohammadi A, Barikani M, Barmar M (2013) Effect of surface modification of Fe3O4 nanoparticles on thermal and mechanical properties of magnetic polyurethane elastomer nanocomposites. J Mater Sci 48:7493–7502. https://doi.org/10.1007/s10853-013-7563-7
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents-a critical review. J Hazard Mater 142:1–53. https://doi.org/10.1016/j.jhazmat.2007.01.006
Mousavi SJ, Parvini M, Ghorbani M (2018) Adsorption of heavy metals (Cu2+ and Zn2+) on novel bifunctional ordered mesoporous silica: optimization by response surface methodology. J Taiwan Inst Chem Eng 84:123–141. https://doi.org/10.1016/j.jtice.2018.01.010
Nagy B, Mânzatu C, Măicăneanu A, Indolean C, Barbu-Tudoran L, Majdik C (2017) Linear and nonlinear regression analysis for heavy metals removal using Agaricus bisporus macrofungus. Arab J Chem 10:S3569–S3579. https://doi.org/10.1016/j.arabjc.2014.03.004
Osińska M (2017) Removal of lead(II), copper(II), cobalt(II) and nickel(II) ions from aqueous solutions using carbon gels. J Sol-Gel Sci Technol 81:678–692. https://doi.org/10.1007/s10971-016-4256-0
Pakade V, Cukrowska E, Darkwa J, Torto N, Chimuka L (2011) Selective removal of chromium (VI) from sulphates and other metal anions using an ion-imprinted polymer. Water SA 37:529–538. https://doi.org/10.4314/wsa.v37i4.11
Pal S, Alocilja EC (2009) Electrically active polyaniline coated magnetic (EAPM) nanoparticle as novel transducer in biosensor for detection of Bacillus anthracis spores in food samples. Biosens Bioelectron 24:1437–1444. https://doi.org/10.1016/j.bios.2008.08.020
Paridah M., Moradbak A, Mohamed A., et al (2016) We are IntechOpen, the world’s leading publisher of open access books built by scientists, for scientists TOP 1%. Intech i:13. https://doi.org/10.5772/57353
Peasura P (2015) Application of response surface methodology for modeling of postweld heat treatment process in a pressure vessel steel ASTM A516 grade 70. Sci World J 2015:1–8. https://doi.org/10.1155/2015/318475
Poinern G, Brundavanam S, Tripathy S et al (2016) Kinetic and adsorption behaviour of aqueous cadmium using a 30 nm hydroxyapatite based powder synthesized via a combined ultrasound and microwave based technique. Phys Chem 6:11–22. https://doi.org/10.5923/j.materials.20150502.02
Qiu H, Lv L, Pan BC, Zhang QJ, Zhang WM, Zhang QX (2009) Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 10:716–724. https://doi.org/10.1631/jzus.A0820524
Rastegar SO, Mousavi SM, Shojaosadati SA, Sheibani S (2011) Optimization of petroleum refinery effluent treatment in a UASB reactor using response surface methodology. J Hazard Mater 197:26–32. https://doi.org/10.1016/j.jhazmat.2011.09.052
Renu, Agarwal M, Singh K (2017) Heavy metal removal from wastewater using various adsorbents: a review. J Water Reuse Desalin 7:387–419. https://doi.org/10.2166/wrd.2016.104
Saadat A, Hajiaghababaei L, Badiei A et al (2019) Amino functionalized silica coated fe3O4 magnetic nanoparticles as a novel adsorbent for removal of pb2+ and cd2+. Pollution 5:847–857. https://doi.org/10.22059/poll.2019.274986.573
Shete SB, Joshi UD (2015) Synthesis and characterization of high quality mesoporous material SBA-16 with ultrasonication. IOSR J Appl Phys 7:50–53. https://doi.org/10.9790/4861-07225053
Song J, Song Z, Sun R (2012) Study of uniform experiment design method applying to civil engineering. Procedia Eng 31:739–745. https://doi.org/10.1016/j.proeng.2012.01.1095
Sulaymon AH, Mohammed TJ, Al-najar J (2012) Equilibrium and kinetics studies of adsorption of heavy metals onto activated carbon. Can J Chem Eng Technol:3
Sun F, Wang Y, Xu H (2019) Uniform projection designs. Ann Stat 47:641–661. https://doi.org/10.1214/18-AOS1705
Tada K, Nagaya Y, Kunieda S, Suyama K, Fukahori T (2017) Development and verification of a new nuclear data processing system FRENDY. J Nucl Sci Technol 54:806–817. https://doi.org/10.1080/00223131.2017.1309306
Talke IS, Borkowski JJ (2012) Generation of space-filling uniform designs in unit hypercubes. J Stat Plan Inference 142:3189–3200. https://doi.org/10.1016/j.jspi.2012.06.013
Tan IAW, Ahmad AL, Hameed BH (2008) Preparation of activated carbon from coconut husk: optimization study on removal of 2,4,6-trichlorophenol using response surface methodology. J Hazard Mater 153:709–717. https://doi.org/10.1016/j.jhazmat.2007.09.014
Venkateswarlu S, Yoon M (2015) Core-shell ferromagnetic nanorod based on amine polymer composite (Fe3O4@DAPF) for fast removal of Pb(II) from aqueous solutions. ACS Appl Mater Interfaces 7:25362–25372. https://doi.org/10.1021/acsami.5b07723
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D (2010) Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349:293–299. https://doi.org/10.1016/j.jcis.2010.05.010
Wang JP, Chen YZ, Wang Y, Yuan SJ, Yu HQ (2011) Optimization of the coagulation-flocculation process for pulp mill wastewater treatment using a combination of uniform design and response surface methodology. Water Res 45:5633–5640. https://doi.org/10.1016/j.watres.2011.08.023
Wu W, Wu Z, Yu T, Jiang C, Kim WS (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16:23501. https://doi.org/10.1088/1468-6996/16/2/023501
Wu D, Wang W, Ng TW, Huang G, Xia D, Yip HY, Lee HK, Li G, An T, Wong PK (2016) Visible-light-driven photocatalytic bacterial inactivation and the mechanism of zinc oxysulfide under LED light irradiation. J Mater Chem A 4:1052–1059. https://doi.org/10.1039/c5ta08044d
Xi Y, Luo Y, Luo J, Luo X (2015) Removal of cadmium(II) from wastewater using novel cadmium ion-imprinted polymers. J Chem Eng Data 60:3253–3261. https://doi.org/10.1021/acs.jced.5b00494
Xin X, Wei Q, Yang J, Yan L, Feng R, Chen G, du B, Li H (2012) Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe 3O 4 nanoparticles. Chem Eng J 184:132–140. https://doi.org/10.1016/j.cej.2012.01.016
Yan Z, Gao J, Li Y, Zhang M, Guo M (2015) Hydrothermal synthesis and structure evolution of metal-doped magnesium ferrite from saprolite laterite. RSC Adv 5:92778–92787. https://doi.org/10.1039/c5ra17145h
Yang H, Fan J, Tian H, Wang X, Fu W, Alam E (2019) Synthesis of imprinted amino-functionalized mesoporous silica and their selective adsorption performance of Pb2+, Cu2+, and Zn2+. J Sol-Gel Sci Technol 90:465–477. https://doi.org/10.1007/s10971-019-04985-6
Zhang J, Zhai S, Li S, Xiao Z, Song Y, An Q, Tian G (2013) Pb(II) removal of Fe3O4@SiO2-NH2 core-shell nanomaterials prepared via a controllable sol-gel process. Chem Eng J 215–216:461–471. https://doi.org/10.1016/j.cej.2012.11.043
Zhu Y, Li L, Wang W et al (2015) Molecularly imprinted polymers on a silica surface for the adsorption of tobacco-specific nitrosamines in mainstream cigarette smoke. Separation. 38:38–2557. https://doi.org/10.1002/jssc.201500193
Zhu W, Wang J, Wu D, Li X, Luo Y, Han C, Ma W, He S (2017) Investigating the heavy metal adsorption of mesoporous silica materials prepared by microwave synthesis. Nanoscale Res Lett 12:12. https://doi.org/10.1186/s11671-017-2070-4
Contribution of authors
EA was involved in methodology, preparation of materials, characterizations, adsorption experiments, and manuscript writing. QF helped in experiment design, supervision, expert feedback, and reviewing. HY contributed to the methodology, supervision, materials, experiment design, and editing. JF helped in uniform design, response surface methodology, and XRD of the materials. SM helped in interpretation of data, proof reading, and revision of the manuscript.
Funding
This study received financial support from the Fundamental Research Funds for the Central Universities by Chinese government (2017QNB05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alam, E., Feng, Q., Yang, H. et al. Synthesis of magnetic core-shell amino adsorbent by using uniform design and response surface analysis (RSM) and its application for the removal of Cu2+, Zn2+, and Pb2+. Environ Sci Pollut Res 28, 36399–36414 (2021). https://doi.org/10.1007/s11356-020-11840-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11840-7