Abstract
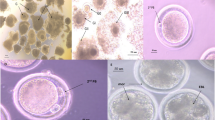
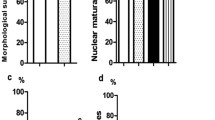
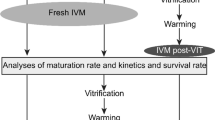
Oocyte vitrification preserves the female genetic resources of elite dromedary camels. In the current study, we aimed to explore the effects of vitrification of camel oocytes on mitochondrial activity, redox stress, and expression of genes related to mitochondrial function, apoptosis, pluripotency, and cytoskeleton. Moreover, we investigated developmental competence of vitrified oocytes after parthenogenetic activation. Oocytes vitrified with the Cryotop method were compared with the fresh oocytes. Our results showed that vitrification led to increased ROS production in oocytes as evidenced by an increase in the DCFDHA fluorescence intensity, and lower mitochondrial activity. At the molecular level, vitrification reduced mRNA expression of many genes, including those related to mitochondrial function (TFAM, MT-CO1, MFN1, ATP1A1, NRF1), pluripotency (SOX2 and POU5F1), and apoptosis (p53 and BAX). In contrast, expression of KLF4 and cytoskeleton-related genes (ACTB and KRT8) was not affected. However, we found no difference in the rates of oocyte survival, cleavage, and blastocyst development, and blastocyst hatching between fresh and vitrified oocytes after warming. Our results indicate that although vitrification of camel metaphase II (MII) oocytes adversely affected mitochondrial functions, the effect was transient without compromising the developmental potential of the oocytes after parthenogenetic activation.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdelkhalek AE, Gabr SA, Khalil WA, Shamiah SM, Pan L, Qin G, Farouk MH (2017) In vitro production of Sudanese camel (Camelus dromedarius) embryos from epididymal spermatozoa and follicular oocytes of slaughtered animals. Pol J Vet Sci 20(1):95–101
Abri MAA, Faye B (2019) Genetic improvement in dromedary camels: challenges and opportunities. Front Genet 10
Antelman J, Manandhar G, Yi YJ, Li R, Whitworth KM, Sutovsky M, Agca C, Prather RS, Sutovsky P (2008) Expression of mitochondrial transcription factor a (TFAM) during porcine gametogenesis and preimplantation embryo development. J Cell Physiol 217(2):529–543
Bonte D, Thys V, De Sutter P, Boel A, Leybaert L, Heindryckx B (2020) Vitrification negatively affects the Ca2+-releasing and activation potential of mouse oocytes, but vitrified oocytes are potentially useful for diagnostic purposes. Reprod BioMed Online 40(1):13–25
Chen S-U, Yang Y-S (2009) Slow freezing or vitrification of oocytes: their effects on survival and meiotic spindles, and the time schedule for clinical practice. Taiwan J Obstet Gynecol 48(1):15–22
Chen C, Han S, Liu W, Wang Y, Huang G (2012) Effect of vitrification on mitochondrial membrane potential in human metaphase II oocytes. J Assist Reprod Genet 29(10):1045–1050
Chian R-C, Wang Y, Li Y-R (2014) Oocyte vitrification: advances, progress and future goals. J Assist Reprod Genet 31(4):411–420
Cobo A, Coello A, Remohí J, Serrano J, de los Santos JM, Meseguer M (2017) Effect of oocyte vitrification on embryo quality: time-lapse analysis and morphokinetic evaluation. Fertil Steril 108(3):491–497.e493
De Munck N, Vajta G (2017) Safety and efficiency of oocyte vitrification. Cryobiology 78:119–127
De Munck N, Petrussa L, Verheyen G, Staessen C, Vandeskelde Y, Sterckx J, Bocken G, Jacobs K, Stoop D, De Rycke M et al (2015) Chromosomal meiotic segregation, embryonic developmental kinetics and DNA (hydroxy) methylation analysis consolidate the safety of human oocyte vitrification. MHR Basic Sci Reprod Med 21(6):535–544
de Oliveira Leme L, Dufort I, Spricigo JFW, Braga TF, Sirard M-A, Franco MM, Dode MAN (2016) Effect of vitrification using the Cryotop method on the gene expression profile of in vitro–produced bovine embryos. Theriogenology 85(4):724–733.e721
De Santis L, Nottola SA, Coticchio G, Borini A, Iussig B, Miglietta S, Macchiarelli G (2020) Type of protein supplement in cryopreservation solutions impacts on the degree of ultrastructural damage in frozen-thawed human oocytes. Cryobiology 95:143–150
Duan X, Sun S-C (2019) Actin cytoskeleton dynamics in mammalian oocyte meiosis†. Biol Reprod 100(1):15–24
Ebrahimi B, Valojerdi MR, Eftekhari-Yazdi P, Baharvand H (2010) In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet 27(5):239–246
El-Badry DA, Scholkamy TH, Darwish GM (2015) Vitrification of dromedary camel oocytes: effect of maturation state, equilibration and vitrification time and type of cryoprotectant. J Egypt Med Assoc 75(1):7–17
Fathi M, Moawad AR, Badr MR (2018) Production of blastocysts following in vitro maturation and fertilization of dromedary camel oocytes vitrified at the germinal vesicle stage. PLoS One 13(3):e0194602
Forman EJ, Li X, Ferry KM, Scott K, Treff NR, Scott RT Jr (2012) Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril 98(3):644–649
Ghaffari Novin M, Allahveisi A, Noruzinia M, Farhadifar F, Yousefian E, Dehghani Fard A, Salimi M (2015) The relationship between transcript expression levels of nuclear encoded (TFAM, NRF1) and mitochondrial encoded (MT-CO1) genes in single human oocytes during oocyte maturation. Balkan J Med Genet 18(1):39–46
Gutnisky C, Morado S, Gadze T, Donato A, Alvarez G, Dalvit G, Cetica P (2020) Morphological, biochemical and functional studies to evaluate bovine oocyte vitrification. Theriogenology 143:18–26
Hosseini SM, Asgari V, Ostadhosseini S, Hajian M, Ghanaei HR, Nasr-Esfahani MH (2015) Developmental competence of ovine oocytes after vitrification: differential effects of vitrification steps, embryo production methods, and parental origin of pronuclei. Theriogenology 83(3):366–376
Huang J, Tan SL, Chian R-C (2006) Fertility preservation for female. J Reprod Contracept 17(2):109–128
Ibrahim MA, Radwan MI, Kim HK, Han J, Warda M (2020) Evaluation of global expression of selected genes as potential candidates for internal normalizing control during transcriptome analysis in dromedary camel (camelus dromedarius). Small Rumin Res 184:106050
Jahangiri M, Shahhoseini M, Movaghar B (2018) The effect of vitrification on expression and histone marks of Igf2 and Oct4 in blastocysts cultured from two-cell mouse embryos. Cell J 19(4):607–613
Khalili MA, Shahedi A, Ashourzadeh S, Nottola SA, Macchiarelli G, Palmerini MG (2017) Vitrification of human immature oocytes before and after in vitro maturation: a review. J Assist Reprod Genet 34(11):1413–1426
Lei T, Guo N, Tan M-h, Y-f L (2014) Effect of mouse oocyte vitrification on mitochondrial membrane potential and distribution. J Huazhong Univ Sci Technol [Med Sci] 34(1):99–102
Leon PMM, Campos VF, Kaefer C, Begnini KR, McBride AJA, Dellagostin OA, Seixas FK, Deschamps JC, Collares T (2011) Expression of apoptotic genes in immature and in vitro matured equine oocytes and cumulus cells. Zygote 21(3):279–285
Martinez-Pastor F, Garcia-Macias V, Alvarez M, Chamorro C, Herraez P, Pd P, Anel L (2006) Comparison of two methods for obtaining spermatozoa from the cauda epididymis of Iberian red deer. Theriogenology 65(3):471–485
Moawad M, Hussein HA, Abd El-Ghani M, Darwish G, Badr M (2019) Effects of cryoprotectants and cryoprotectant combinations on viability and maturation rates of Camelus dromedarius oocytes vitrified at germinal vesicle stage. Reprod Domest Anim 54(1):108–117
Monzo C, Haouzi D, Roman K, Assou S, Dechaud H, Hamamah S (2012) Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Hum Reprod 27(7):2160–2168
Moulavi F, Hosseini SM (2018) Diverse patterns of cumulus cell expansion during in vitro maturation reveal heterogeneous cellular and molecular features of oocyte competence in dromedary camel. Theriogenology 119:259–267
Moulavi F, Hosseini SM (2019) Effect of macromolecule supplement on nuclear and cytoplasmic maturation, cryosurvival and in vitro embryo development of dromedary camel oocytes. Theriogenology 132:62–71
Moulavi F, Soto-Rodriguez S, Kuwayama M, Asadi-Moghaddam B, Hosseini S-M (2019) Survival, re-expansion, and pregnancy outcome following vitrification of dromedary camel cloned blastocysts: a possible role of vitrification in improving clone pregnancy rate by weeding out poor competent embryos. Cryobiology 90:75–82
Nohales-Córcoles M, Sevillano-Almerich G, Di Emidio G, Tatone C, Cobo AC, Dumollard R, De los Santos Molina MJ. (2016) Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Hum Reprod 31(8):1850–1858
Nottola SA, Albani E, Coticchio G, Palmerini MG, Lorenzo C, Scaravelli G, Borini A, Levi-Setti PE, Macchiarelli G (2016) Freeze/thaw stress induces organelle remodeling and membrane recycling in cryopreserved human mature oocytes. J Assist Reprod Genet 33(12):1559–1570
Palmerini MG, Antinori M, Maione M, Cerusico F, Versaci C, Nottola SA, Macchiarelli G, Khalili MA, Antinori S (2014) Ultrastructure of immature and mature human oocytes after cryotop vitrification. J Reprod Dev 60(6):411–420
Rienzi L, Cobo A, Ubaldi FM (2017) Chapter 10 human oocyte vitrification. 1568:131-139
Russo R, Monaco D, Rubessa M, El-Bahrawy KA, El-Sayed A, Martino NA, Beneult B, Ciannarella F, Dell’Aquila ME, Lacalandra GM et al (2014) Confocal fluorescence assessment of bioenergy/redox status of dromedary camel (Camelus dromedarius) oocytes before and after in vitro maturation. Reprod Biol Endocrinol 12(1):16
Saadeldin IM, Swelum AA-A, Elsafadi M, Moumen AF, Alzahrani FA, Mahmood A, Alfayez M, Alowaimer AN (2017a) Isolation and characterization of the trophectoderm from the Arabian camel (Camelus dromedarius). Placenta 57:113–122
Saadeldin IM, Swelum AA-A, Yaqoob SH, Alowaimer AN (2017b) Morphometric assessment of in vitro matured dromedary camel oocytes determines the developmental competence after parthenogenetic activation. Theriogenology 95:141–148
Saadeldin IM, Swelum AA-A, Elsafadi M, Mahmood A, Alfayez M, Alowaimer AN (2018) Differences between the tolerance of camel oocytes and cumulus cells to acute and chronic hyperthermia. J Therm Biol 74:47–54
Saragusty J, Arav A (2011) Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 141(1):1–19
Singh B, Mal G, Gautam SK, Mukesh M (2019) Reproduction biotechnology in Camelids. 145-153
Skidmore JA (2003) The main challenges facing camel reproduction research in the 21st century. Reprod Suppl 61:37–47
Skidmore JA (2019) The use of some assisted reproductive technologies in old world camelids. Anim Reprod Sci 207:138–145
Somfai T, Imai K, Kaneda M, Akagi S, Watanabe S, Haraguchi S, Mizutani E, Dang-Nguyen TQ, Inaba Y, Geshi M et al (2011) The effect of ovary storage and in vitro maturation on mRNA levels in bovine oocytes; a possible impact of maternal ATP1A1 on blastocyst development in slaughterhouse-derived oocytes. J Reprod Dev 57(6):723–730
Song W-Y, Peng Z-F, Chen X-M, Jin H-X, Yao G-D, Shi S-L, Yang H-Y, Zhang X-Y, Sun Y-P (2016) Effects of vitrification on outcomes of in VivoMature, in vitro-mature and immature human oocytes. Cell Physiol Biochem 38(5):2053–2062
Spinaci M, Vallorani C, Bucci D, Tamanini C, Porcu E, Galeati G (2012) Vitrification of pig oocytes induces changes in histone H4 acetylation and histone H3 lysine 9 methylation (H3K9). Vet Res Commun 36(3):165–171
Vajta G (2000) Vitrification of the oocytes and embryos of domestic animals. Anim Reprod Sci 60-61:357–364
Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, Callesen H (1998) Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev 51(1):53–58
Wang N, Hao H-S, Li C-Y, Zhao Y-H, Wang H-Y, Yan C-L, Du W-H, Wang D, Liu Y, Pang Y-W et al (2017a) Calcium ion regulation by BAPTA-AM and ruthenium red improved the fertilisation capacity and developmental ability of vitrified bovine oocytes. Sci Rep 7(1):10652
Wang N, Li CY, Zhu HB, Hao HS, Wang HY, Yan CL, Zhao SJ, Du WH, Wang D, Liu Y et al (2017b) Effect of vitrification on the mRNA transcriptome of bovine oocytes. Reprod Domest Anim 52(4):531–541
Wei X, Sijie Y, Weibin Z, Qing X, Jie Z, Xiangdong Z (2017) Cytoskeleton genes expression and survival rate comparison between immature and mature yak oocyte after OPS vitrification. Anim Biotechnol 29(4):247–251
Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G (2001) Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 16(5):909–917
Yan C-L, Fu X-W, Zhou G-B, Zhao X-M, Suo L, Zhu S-E (2010a) Mitochondrial behaviors in the vitrified mouse oocyte and its parthenogenetic embryo: effect of Taxol pretreatment and relationship to competence. Fertil Steril 93(3):959–966
Yan X, Yu S, Lei A, Hua J, Chen F, Li L, Xie X, Yang X, Geng W, Dou Z (2010b) The four reprogramming factors and embryonic development in mice. Cell Reprogram 12(5):565–570
Yaqoob SH, Saadeldin IM, Swelum AA-A, Alowaimer AN (2017) Optimizing camel (Camelus dromedarius) oocytes in vitro maturation and early embryo culture after parthenogenetic activation. Small Rumin Res 153:81–86
Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, Seli E (2018) MFN1 is required for follicle development, oocyte maturation, and female fertility. Fertil Steril 110(4):e27–e28
Zhen X, Wu B, Wang J, Lu C, Gao H, Qiao J (2015) Increased incidence of mitochondrial cytochrome C oxidase 1 gene mutations in patients with primary ovarian insufficiency. PLoS One 10(7):e0132610
Acknowledgments
The authors are highly thankful to Mr. Hamad Buti Mohammed, the General Director of CARTC, for their moral and unconditional support. Authors thank Embryology lab staff, for assistance in this study, and administrative office, for arrangements. The authors would like to thank the Deanship of Scientific Research at King Saud University through research group RG-1438-018 for partial funding of this work.
Funding
This study was kindly sponsored by Zaabeel Office for H. H. Sheikh Mohammed Bin Rashid Al Maktoum, Vice President and Prime Minister of the UAE and ruler of Dubai. This study was partially funded by the Deanship of Scientific Research at King Saud University through research group RG-1438-018.
Author information
Authors and Affiliations
Contributions
Conceptualization, I.M.S., M.F., and S.M.H.; methodology, M.F., I.M.S., A.A.S., and S.M.H.; investigation, M.F., I.M.S., and S.M.H; data curation, M.F., I.M.S., A.A.S., K.S.S., H.F.H., and S.M.H.; writing—review and editing, I.M.S. and S.M.H.; supervision, I.M.S. and S.M.H.; project administration, I.M.S., S.M.H.; funding acquisition, I.M.S. and S.M.H.. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The effects of vitrification on camel MII oocytes have been studied.
• Vitrification adversely affected mitochondrial protein and mRNA transcripts expression.
• The adverse effect was transient and did not affect the developmental potential of oocytes after ICSI and parthenogenetic activation.
Rights and permissions
About this article
Cite this article
Saadeldin, I.M., Moulavi, F., Swelum, A.AA. et al. Vitrification of camel oocytes transiently impacts mitochondrial functions without affecting the developmental potential after intracytoplasmic sperm injection and parthenogenetic activation. Environ Sci Pollut Res 27, 44604–44613 (2020). https://doi.org/10.1007/s11356-020-11070-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11070-x