Abstract
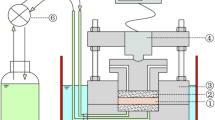
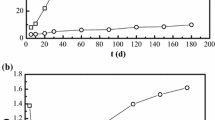
In the Chinese high-level radioactive waste geological disposal program, Gaomiaozi (GMZ) bentonite has been selected as the potential buffer/backfill material. After the closure of the repository, the Ca-OH-type alkaline solution (evolved cement water) released by cement degradation may last for more than 100,000 years. The bentonite will undergo the corrosion of evolved cement water (ECW) for a long period. This work focuses on the sorption property of GMZ bentonite altered by ECW. Firstly, the corrosion experiments on compacted GMZ specimens with the dry density of 1.70 Mg/m3 were carried out under constant volume conditions at two temperatures. Then, the sorption of europium (Eu (III)) onto the corroded GMZ bentonite was studied by batch experiments. The results of batch sorption tests indicate that the altered GMZ bentonite keeps an effective removal property with the uptake of Eu (III) more than 99%. The effect of high-temperature conditions of the repository on the sorption property of bentonite is not significant. The results also suggest that the evolved cement water presents no detrimental effect on the long-term adsorption performance of bentonite even under higher temperature conditions.












Similar content being viewed by others
References
Abdel Raouf MW, El-Kamash AM (2006) Kinetics and thermodynamics of the sorption of uranium and thorium ions from nitric acid solutions onto a TBP impregnated sorbent. J Radioanal Nucl Chem 267:389–395
Aksoyoglu S (1989) Sorption of U (VI) on granite. J Radioanal Nucl Chem 134:393–403
Baeyens B, Bradbury MH (1997) A mechanistic description of Ni and Zn sorption on Na-montmorillonite. Part I: Titration and sorption measurements. J Contam Hydrol 27:199–222
Berner UR (1992) Evolution of pore water chemistry during degradation of cement in a radioactive waste repository environment. Waste Manag 12:201–219
Bradbury MH, Baeyens B (2002) Sorption of Eu on Na- and Ca-montmorillonites: experimental investigations and modelling with cation exchange and surface complexation. Geochim Cosmochim Acta 66(13):2325–2334
Chen ZS, Lu SS (2016) Investigation of the effect of pH, ionic strength, foreign ions, temperature, soil humic substances on the sorption of 152+154Eu(III) onto NKF-6 zeolite. J Radioanal Nucl Chem 309:717–728
Chen YG, Ye WM, Yang MX, He Y (2011) Effect of contact time, pH, and ionic strength on Cd (II) adsorption from aqueous solution onto bentonite from Gaomiaozi. China Environ Earth Sci 64(2):329–336
Chen YG, Zhu BH, Wu DB, Wang QG, Yang YH, Ye WM, Guo JF (2012) Eu (III) adsorption using di (2-thylhexly) phosphoric acid-immobilized magnetic GMZ bentonite. Chem Eng J 181-182:387–396
Chen YG, Zhu CM, Ye WM, Cui YJ, Wang Q (2015) Swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite under salinization-desalinization cycle conditions. Appl Clay Sci 114:454–460
Chen YG, Zhu CM, Ye WM, Cui YJ, Chen B (2016) Effects of solution concentration and vertical stress on the swelling behavior of compacted GMZ01 bentonite. Appl Clay Sci 124:11–20
Chen YG, Sun Z, Ye WM, Cui YJ (2017) Adsorptive removal of Eu (III) from simulated groundwater by GMZ bentonite on the repository conditions. J Radioanal Nucl Chem 311(3):1839–1847
Chen YG, Sun Z, Cui YJ, Ye WM, Liu QH (2019) Effect of cement solutions on the swelling pressure of compacted GMZ bentonite at different temperatures. Constr Build Mater 229:116872
Cuevas J, Ruiz AI, Fernandez R (2018) Investigating the potential barrier function of nanostructured materials formed in engineered barrier systems (EBS) designed for nuclear waste isolation. Chem Reccord 18:1065–1075
Cuevas J, Ruiz AI, Fernández R, Torres E, Escribano A, Regadío M, Turrero MJ (2016) Lime mortar-compacted bentonite–magnetite interfaces: an experimental study focused on the understanding of the EBS long-term performance for high-level nuclear waste isolation DGR concept. Appl Clay Sci 124–125:79–93
Donat R, Akdogan A, Erdem E, Cetisli H (2005) Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. J Colloid Interface Sci 286:43–52
Erdem B, Özcan A, Gök Ö, Özcan AS (2009) Immobilization of 2, 2′-dipyridyl onto bentonite and its adsorption behavior of copper (II) ions. J Hazard Mater 163:418–426
European Commission, 2005. ECOCLAY II: effects of cement on clay barrier performance – phase II ECOCLAY II – FIKW-CT-2000-00018, Final Report
Faucon P, Adenot F, Jacquinot JF, Petit JC, Cabrillac R, Jorda M (1998) Long-term behavior of cement pastes used for nuclear waste disposal: review of physico-chemical mechanisms of water degradation. Cem Concr Res 28(6):847–857
Fernández R, Mäder UK, Rodríguez M, Vigil de la Villa R, Cuevas J (2009) Alteration of compacted bentonite by diffusion of highly alkaline solutions. Eur J Mineral 21:725–735. https://doi.org/10.1127/0935-1221/2009/0021-1947
Fernández R, Ruiz AI, Cuevas J (2014) The role of smectite composition on the hyperalkaline alteration of bentonite. Appl Clay Sci 95:83–94
Garcia SG, Jonsson M, Wold S (2006) Temperature effect on the stability of bentonite colloids in water. J Colloid Interface Sci 298:694–705
Guo YH, Yang TX, Liu SF (2001) Hydrogeological characteristics of Beishan preselected area, Gansu province for China’s high-level radioactive waste repository. Uranium Geol 3(17):184–189
Guo YH, Li YW, Wang HL, Su R, Liu SF, Zong ZH (2010) Study on regional hydrogeochemical characteristics of Beishan area—the preselected area for China’s high level radioactive waste repository. Proceeding of the 3rd Conference on Underground Disposal of the Waste 19–24 (in Chinese)
He Y, Chen Y, Ye W (2016) Equilibrium, kinetic, and thermodynamic studies of adsorption of Sr (II) from aqueous solution onto GMZ bentonite. Environ Earth Sci 75:2–10
Heath TG, Hunter FMI (2012) Calculation of near field pH buffering: effect of polymer encapsulant. Serco Report SA/ENV-0909 Issue 2
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. J Environ Sci Health B 76(B2):183–191
Hökmark H, Claesson J (2005) Use of an analytical solution for calculating temperatures in repository host rock. Eng Geol 81:353–364
Hu J, Xie Z, He B, Sheng GD, Chen CL, Li JX, Chen YX, Wang XK (2010) Sorption of Eu (III) on GMZ bentonite in the absence/presence of humic acid studied by batch and XAFS techniques. Science China Chem 53(6):1420–1428
Hummel W, Berner U, Curti E, Thoenen A (2002) Update of the Nagra/PSI thermodynamic data base. Nagra Technical Report, Nagra
Johnson JW, Oelkers EH, Helgeson HC (1992) SUPCRT92: a software package for calculating the standard molars thermodynamic properties of minerals; gases, aqueous species and reactions from 1 to 5000 bars and 0 to 1000 C. Comput Geosci 18:899–947
Karnland O, Olsson S, Nilsson U, Sellin P (2007) Experimentally determined swelling pressures and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Physics and Chemistry of the Earth, Parts A/B/C 32(1–7):275–286
Kitayama K, Ueda H, Sasaki N, Hironaga M (2004) General overview on the use of cement in repositories for HLW and other radioactive wastes. Proceedings of the International Workshop on Bentonite-Cement Interaction in Repository Environments. Tokyo, Japan
Lee JO, Kang IM, Cho WJ (2010) Smectite alteration and its influence on the barrier properties of smectite clay for a repository. Appl Clay Sci 47:99–104
Liu YM, Xu GQ, Liu SF (2001) Study on the basic property of Gaomiaozi bentonite, Inner Mongolia. China Nuclear Industry Audio & Visual Publishing House, Beijing, 1–20 (in Chinese)
Liu FQ, Ye YL, Guo N, Zhang R, Wu WS, Guo ZJ (2013) The adsorption of Eu (III) on Gaomiaozi Na-bentonite: experimental and modeling study. Sci Sin Chim 43(2):242–252
Lu SS, Xu H, Wang MM, Song XP, Liu Q (2012) Sorption of Eu (III) onto Gaomiaozi bentonite by batch technique as a function of pH, ionic strength and humic acid. J Radioanal Nucl Chem 292:889–895
Lu ZH, Hao ZQ, Wang J, Chen L (2016) Efficient removal of europium from aqueous solutions using attapulgite-iron oxide magnetic composites. J Ind Eng Chem 34:374–381
Meunier A, Proust D, Beaufort D (1992) Heterogeneous reactions of dioctahedral smectites in illite-smectite and kaolinite-smectite mixed-layers: applications to clay materials for engineered barriers. Appl Geochem Suppl 1:143–150
NEA (2003) Update on the chemical thermodynamics of uranium, neptunium, plutonium, americium and technetium. In Chemical Thermodynamics (ed. OECD Nuclear Energy Agency). Elsevier, Amsterdam, vol. 5
Nguyena TS, Selvadura APS, Armand G (2005) Modelling the FEBEX THM experiment using a state surface approach. Int J Rock Mech Min Sci 42:639–651
Partey F, Norman D, Ndur S, Nartey R (2008) Arsenic sorption onto laterite iron concretions: temperature effect. J Colloid Interface Sci 321:493–500
Ramirez S, Vieillard P, Bouchet A, Cassagnabère A, Meunier A, Jacquot E (2005) Alteration of the Callovo-Oxfordian Clay from Meuse-Haute Marne underground laboratory (France) by alkaline solution. I. A XRD and CEC study. Appl Geochem 20:89–99
Reddad Z, Gerente C, Andres Y, Cloirec LP (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Samper J, Montenegro L, Turrero MJ, Martín PL, Garralón A, Cuevas J, Fernández R (2010) Technical note 1: design of new experiments. PEBS Internal Deliverable D 3.4.2. 10 pp
Savage D (1997) Review of the potential effects of alkaline plume migration from a cementitious repository for radioactive waste. Implications for Performance Assessment (Environment Agency, UK), R&D Technical Report, p60
Savage D, Noy D, Mihara M (2002) Modelling the interaction of bentonite with hyperalkaline fluids. Appl Geochem 17:207–223
Savage D, Walker C, Arthur R, Rochelle C, Oda C, Takase H (2007) Alteration of bentonite by hyperalkaline fluids: a review of the role of secondary minerals. Phys Chem Earth 32:287–297
Shao DD, Xu D, Wang SW, Fan QH, Wu WS, Dong YH, Wang XK (2009) Modeling of radionickel sorption on MX-80 bentonite as a function of pH and ionic strength. Sci China Series B Chem 52:362–371
Sheng GD, Shao DD, Fan QH, Xu D, Chen YX, Wang XK (2009) Effect of pH and ionic strength on sorption of Eu (III) to MX-80 bentonite: batch and XAFS study. Radiochim Acta 97:621–630. https://doi.org/10.1524/ract.2009.1656
Sun YB, Li JX, Wang XK (2014) The retention of uranium and europium onto sepiolite investigated by macroscopic, spectro-scopic and modeling techniques. Geochim Cosmochim Acta 140:621–643
Sun Z, Chen YG, Cui YJ, Xu HD, Ye WM, Wu DB (2018) Effect of synthetic water and cement solutions on the swelling pressure of compacted Gaomiaozi (GMZ) bentonite: the Beishan site case, Gansu, China. Eng Geol 244:66–74
Sun Z, Chen YG, Cui YJ, Ye WM (2019) Effect of synthetic Beishan site water and cement solutions on the mineralogy and microstructure of compacted GMZ bentonite. Soils Found 59:2056–2069. https://doi.org/10.1016/j.sandf.2019.11.006
Sun Z, Chen YG, Cui YJ, Ye WM (2020) Adsorption of Eu (III) onto Gaomiaozi bentonite corroded by cement waters: effect of cement solutions on the long-term sorption performance of bentonite in the repository conditions. J Clean Prod 251:119692. https://doi.org/10.1016/j.jclepro.2019.119692
Tertre E, Berger G, Tertre E, Berger G, Simoni E, Castet S, Giffaut E, Loubet M, Catalette H (2006a) Europium retention onto clay minerals from 25 to 150 °C: experimental measurements, spectroscopic features and sorption modelling. Geochim Cosmochim Acta 70(18):4563–4578
Tertre E, Castet S, Berger G, Loubet M, Giffaut E (2006b) Surface chemistry of kaolinite and Na-montmorillonite in aqueous electrolyte solutions at 25 and 60 °C: experimental and modeling study. Geochim Cosmochim Acta 70:4579–4599
Vijaya Y, Popuri SR, Boddu VM, Krishnaiah A (2008) Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr Polym 72:261–271
Wang J, Chen WM (2006) Geological disposal of high-level radioactive waste and its key scientific issues. Chin J Rock Mech Eng 25:801–812 (in Chinese)
Wang XK, Chen CL, Zhou X, Tan XL, Hu WP (2005) Diffusion and sorption of U (VI) in compacted bentonite studied by a capillary method. Radiochim Acta 93:273–278
Wang S, Li H, Xu L (2006) Application ofzeolite MCM-22 for basic dye removal from wastewater. J Colloid Interface Sci 295(1):71–78
Wang SW, Dong YH, He ML, Chen L, Yu XJ (2009) Characterization of GMZ bentonite and its application in the adsorption of Pb (II) from aqueous solutions. Appl Clay Sci 43:164–171
Wang L, Jacques D, P De Cannière (2010) Effects of an alkaline plume on the Boom Clay as a potential host formation for geological disposal of radioactive waste. SCK •CEN - ER- 28, Belgium
Wang XX, Sun YB, Alsaedi A, Hayat T, Wang XK (2015) Interaction mechanism of Eu (III) with MX-80 bentonite studied by batch, TRLFS and kinetic desorption techniques. Chem Eng J 264:570–576
Weber WJ, Morriss JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng 89:31–60
Wood SA (1990) The aqueous geochemistry of the rare-earth elements and yttrium. 2. Theoretical predictions of speciation in hydrothermal solutions to 350 C at saturation water vapor pressure. Chem Geol 88:99–125
Xu D, Xu XL, Tan XL, Chen CL, Wang XK (2008) Adsorption of Pb (II) from aqueous solution to MX-80 bentonite: effect of pH, ionic strength, foreign ions and temperature. Appl Clay Sci 41:37–46
Ye WM, Schanz T, Qian LX, Wang J, Arifin YF (2007) Characteristics of swelling pressure of densely compacted gaomiaozi bentonite GMZ01. Chin J Rock Mech Eng 26:3861–3865
Ye WM, Cui YJ, Qian LX, Chen B (2009) An experimental study of the water transfer through confined compacted GMZ bentonite. Eng Geol 108:169–176
Ye WM, Chen YG, Chen B, Cui YJ, Wang J (2010) Advances on the knowledge of the buffer/backfill properties of heavily-compacted GMZ bentonite. Eng Geol 116:12–20
Ye WM, Zheng ZJ, Chen B, Chen YG, Cui YJ, Wang J (2014) Effects of pH and temperature on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Appl Clay Sci 101:192–198
Zhao DL, Feng SJ, Chen CL, Chen SH, Xu D, Wang XK (2008) Adsorption of thorium (IV) on MX-80 bentonite: effect of pH ionic strength and temperature. Appl Clay Sci 41:17–23
Zhao DL, Chen SH, Yang SB, Yang X, Yang ST (2011) Investigation of the sorption behavior of Cd (II) on GMZ bentonite as affected by solution chemistry. Chem Eng J 166:1010–1016
Zhu SJ, Hou HB, Xue YJ (2008) Kinetic and isothermal studies of lead ion adsorption onto bentonite. Appl Clay Sci 40:171–178
Zhu CM, Ye WM, Chen YG, Chen B, Cui YJ (2015) Impact of cyclically infiltration of CaCl2 solution and de-ionized water on volume change behavior of compacted GMZ01 bentonite. Eng Geol 184:104–110
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (41772279 & 41977232) and Fundamental Research Funds for the Central Universities. The authors also wish to acknowledge the support of the European Commission via the Marie Curie IRSES project GREAT “Geotechnical and geological Responses to climate change: Exchanging Approaches and Technologies on a world-wide scale” (FP7-PEOPLE-2013-IRSES-612665).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Z., Chen, Yg., Shang, Y. et al. The sorption performance of corroded Gaomiaozi bentonite by evolved cement water at different temperatures: the case of europium removal. Environ Sci Pollut Res 27, 25057–25068 (2020). https://doi.org/10.1007/s11356-020-08895-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08895-x