Abstract
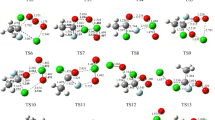
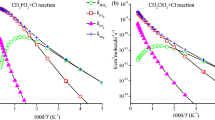
A global and systematic theoretical research on the singlet and triplet potential energy surfaces (PESs) of the CH2ClO2/CHCl2O2 with ClO reactions are done at the CCSD(T)//B3LYP level and accompanied with RRKM computations to forecast the mechanism and distribution of products. The simulation results revealed that, on the singlet PES, products P1 (CHClO + HO2 + Cl)/P1 (CCl2O + HO2 + Cl) from IM1 (CH2ClOOOCl)/IM1 (CHCl2OOOCl) are forecasted to the primary products of the CH2ClO2/CHCl2O2 + ClO reactions, which are initiated by the oxygen atom of ClO radical addition to the terminal-O atom of CH2ClO2/CHCl2O2 barrierlessly, while other product channels contribute less to the whole reactions owing to higher barriers. Two other isomers, including IM2 (CH2ClOOClO) and IM3 (CH2ClOClO2) for the CH2ClO2 + ClO reaction, and three other isomers, including IM2 (CHCl2OOClO), IM3 (CHCl2OClO2), and IM4 (CHCl2ClO3) for the CHCl2O2 + ClO reaction, could be produced as less significant products. RRKM calculations presented that the initial adducts IM1 (CH2ClOOOCl)/IM1 (CHCl2OOOCl) are the primary products at T < 400 K and T < 600 K, respectively, and products P1 (CHClO + HO2 + Cl)/P1 (CCl2O + HO2 + Cl) are dominant the reactions at T ≥ 400 K and T ≥ 600 K, respectively. The atmospheric lifetime of CH2ClO2 and CHCl2O2 in ClO is around 4.61 and 3.24 h, respectively.







Similar content being viewed by others
References
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648
Basco N, Hunt JE (1979) Mutual combination of ClO radicals. Int J Chem Kinet 11(6):649–664
Biggs P, Canosa-Mas CE, Fracheboud JM, Shallcross DE, Wayne RP (1995) Efficiency of formation of CH3O in the reaction of CH3O2 with ClO. Geophys Res Lett 22:1221–1224
Bloss WJ, Nickolaisen SL, Salawitch RJ, Friedl RR, Sander SP (2001) Kinetics of the ClO self-reaction and 210 nm absorption cross section of the ClO dimer. J Phys Chem A 105:11226–11239
Catoire V, Lesclaux R, Schneider WF, Wallington TJ (1996) Kinetics and mechanisms of the self-reactions of CCl3O2 and CHCl2O2 radicals and their reactions with HO2. J Phys Chem 100(34):14356–14371
Cattell FC, Cox RA (1986) Pressure dependence of the reaction of HO2 with Cl and ClO. J Chem Soc Faraday Trans 2, 82: 1413
Chang CT, Liu TH, Jeng FT (2004) Atmospheric concentrations of the Cl atom, ClO radical in the coastal marine boundary layer. Environ Res 94:67–74
Debananda D, Scott LW (1999) Ab initio and density functional theory study of the geometric structure, vibrational frequency, torsional potential, and isomerization of dichlorodisulfane (ClSSCl). J Phys Chem A 103:2134–2140
DeMore WB (1991) Tests of stratospheric models: the reactions of atomic chlorine with O3 and CH4 at low temperature. J Geophys Res 96:4995–5000
Drougas E, Jalbout AF, Kosmas AM (2003) Quantum mechanical studies of CH3ClO3 isomers and the CH3O2 + +ClO reaction pathways. J Phys Chem A 107:11386–11390
Frisch MJ, Trucks GW, Schlegel HB, Gill PWM, Johnson BG, Robb MA, Cheeseman JR, Keith TA, Petersson GA, Pople JA (2009) Gaussian 09. Gaussian, Inc., Wallingford
Galano A, Alvarez-Idaboy JR (2008) Branching ratios of aliphatic amines + OH gas-phase reactions: a variational transition-state theory study. J Chem Theory Comput 4:322–327
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338
Haynes BS (1977) The oxidation of hydrogen cyanide in fuel-rich flames. Combust Flame 28:113–121
Hellei F, Crowley JN, Moortgat GK (1993) Temperature-dependent rate constants and product branching ratios for the gas-phase reaction between CH3O2 and ClO. J Phys Chem 97:11464–11473
Hickson KM, Keyser LF, Sander SP (2007) Temperature dependence of the HO2 + ClO reaction. 2. Reaction kinetics using the discharge-flow resonance-fluorescence technique. J Phys Chem A 111:8126–8138
Holbrook KA, Pilling, MJ, Robertson SH (1996) Unimolecular reactions; J. Wiley: Chichester
Hou H, Wang BS, Gu YS (2000) Ab initio mechanism and multichannel RRKM-TST rate constant for the reaction of Cl(2P) with CH2CO (ketene). J Phys Chem A 104:320–328
Hou H, Wang BS (2007) Ab initio study of the reaction of propionyl (C2H5CO) radical with oxygen (O2). J Chem Phys 127:054306
Klippenstein SJ (1990) Implementation of RRKM theory for highly flexible transition states with a bond length as the reaction coordinate. Chem Phys Lett 170:71–77
Jalbout AF (2002) The isomerization of ClOOCl: high level ab initio and density functional theory analysis. J Mol Struct THEOCHEM 594(1–2):1–7
Kosmas AM, Drougas E (2009) A computational investigation of the atmospheric reaction CH3O2 + ClO. Chem Phys 358:230–234
Kukui AS, Jungkamp TPW, Schindler RN (1994) Determination of the rate constant and of product branching ratios in the reaction of CH3O2 with OCl between 233 and 300 K. Ber Bunsenges Phys Chem 98:1298–1302
Knight GP, Beiderhase T, Helleis F, Moortgat GK, Crowley JN (2000) Reaction of HO2 with ClO: flow tube studies of kinetics and product between 215 and 298 K. J Phys Chem A 104:1674–1685
Leather KE, Bacak A, Wamsley R, Archibald AT, Husk A, Shallcross DE, Percival CJ (2012) Temperature and pressure dependence of the rate coefficient for the reaction between ClO and CH3O2 in the gas-phase. Phys Chem Chem Phys 14:3425–3434
Leck TJ, Jac-EL C, Birks JW (1980) Studies of reactions of importance in the stratosphere. III. Rate constant and products of the reaction between ClO and HO2 radicals at 298 K. J Chem Phys 72:2364
Lee C, Yang W, Par RG (1988) Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys Rev B 37:785
Lee TJ, Taylor PR (1989) A diagnostic for determining the quality of single-reference electron correlation methods. Int J Quantum Chem S23:199–207
Li HW, Tang YZ, Wang RS (2013) Atmospheric chemistry of CF3O2: a theoretical study on mechanisms and pathways of the CF3O2 + IO reaction. Phys Chem Chem Phys 65(16):164–170
Lu CG, Tang YZ, Li DW, Feng J, Sun JY, Zhang YJ, Lv M (2018) Theoretical investigations on the atmospheric C2H5O2 + ClO reaction. Comput Theor Chem 1130:113–120
Miller JA, Bowman CT (1989) Mechanism and modeling of nitrogen chemistry in combustion. Prog Energy Combust Sci 15(4):287–338
Mousavipour SH, Ramazani S, Shahkolahi Z (2009) Multichannel RRKM-TST and direct-dynamics VTST study of the reaction of hydroxyl radical with furan. J Phys Chem A 113:2838
Nicholaisen SL, Friedl RR, Sander SP (1994) Kinetics and mechanism of the chlorine oxide ClO + ClO reaction: pressure and temperature dependences of the bimolecular and termolecular channels and thermal decomposition of chlorine peroxide. J Phys Chem 98:155–169
Nickolaisen SL, Roehl CM, Blakeley LK, Friedl RR, Francisco JS, Liu R, Sander SP (2000) Temperature dependence of the HO2 + ClO reaction. 1. Reaction kinetics by pulsed photolysis-ultraviolet absorption and ab initio studies of the potential surface. J Phys Chem A 104:308–319
Peiró-García J, Nebot-Gil I (2003a) Ab initio study on the mechanism of the atmospheric reaction OH + O3→HO2 + O2. Chem Phys Chem 4:843–847
Peiró-García J, Nebot-Gil I (2003b) Ab initio study of the mechanism of the atmospheric reaction: NO2 + O3→NO3 + O2. J Comput Chem 24:1657–1663
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electron correlation theories. Chem Phys Lett 157:479–483
Ralf S, Yana D, Karol M, Roland HH, Wolffram K (1977) How unstable are Thiosulfoxides? An ab initio MO study of various disulfanes RSSR (R = H, Me, Pr, All), their branched isomers R2S=S, and the related transition states. J Am Chem Soc 119:1990–1996
Rayez MT, Rayez JC, Sawerysyn JP (1994) Ab initio studies of the reactions of chlorine atoms with fluoro- and chloro-substituted methanes. J Phys Chem 98:11342–11352
Reimann B, Kaufman F (1978) Rate constant of the reaction HO2 + ClO → HOCl + O2. J Chem Phys 69:2925–2926
Rienstra-Kiracofe JC, Allen WD, Schaefer HF (2000) The C2H5 + O2 reaction mechanism: high-level ab initio characterizations. J Phys Chem A 104:9823–9840
Robertson S, Wagner AF, Wardlaw DM (2002) Flexible transition state theory for a variable reaction coordinate: analytical expressions and an application. J Phys Chem A 106:2598–2613
Rudolf J (1998) Bonding properties of chlorine oxides ClOx (x=1–4) in the density functional theory. J Mol Struct (THEOCHEM) 423:219–224
Schuchmann HP, Laidler KJ (1972) Nitrogen compounds other than NO in automobile exhaust gas. J Air Pollut Control Assoc 22(1):52–53
Sehested J, Nielsen OJ (1993) Absolute rate constants for the reaction of NO with a series of peroxy radicals in the gas phase at 295 K. Chem Phys Lett 213:457–464
Stolarski RS, Cicerone RJ (1974) Stratospheric chlorine: a possible sink for ozone. Can J Chem 52(8):1610–1615
Sun JY, Wang RS, Wang BS (2011) Theoretical study on the gas phase reaction of acrylonitrile with a hydroxyl radical. Phys Chem Chem Phys 13:16585
Talhaoui A, Louis F, Devolder P, Meriaux B, Sawerysyn JP, Rayez MT, Rayez JC (1996) Rate coefficients of the reactions of chlorine atoms with haloethanes of type CH3CCl3-xFx (x = 0, 1, and 2): experimental and ab initio theoretical studies. J Phys Chem 100:13531–13538
Tang YZ, Sun HF, Sun JY, Zhang YJ, Wang RS (2014) Theoretical study on mechanisms and pathways of the CF3O2 + ClO reaction. Atmos Environ 92:367–375
Tang YZ, Zhang W, Song ZW, Wang XY, Sun JY, Wang RS (2015) Theoretical study on mechanisms and pathways of the atmospheric CF3O2 + BrO reaction. J Fluor Chem 180:110–116
Wang J, Tang YZ, Lu CG, Zhang W, Sun JY, Wang RS (2016) Computational study on mechanisms and pathways of the atmospheric C2H5O2 + IO reaction. Comput Theor Chem 1090:153–156
Wardlaw DM, Marcus RA (1988) On the statistical theory of unimolecular processes. Adv Chem Phys 70:231–263
Xu ZF, Zhu R, Lin MC (2003) Ab initio studies of ClOx reactions. VI. Theoretical prediction of total rate constant and product branching probabilities for the OH2 + ClO reaction. J Phys Chem A 107:3841–3850
Zhang YJ, Sun JY, Chao K, Sun H, Wang F, Tang SW, Pan XM, Zhang JP, Wang RS (2012) Mechanistic and kinetic study of CF3CH=CH2 + OH reaction. J Phys Chem A 116:3172
Zhang YJ, Chao K, Sun JY, Su ZM, Pan XM, Zhang JP, Wang RS (2013) Theoretical study on the gas phase reaction of Allyl alcohol with hydroxyl radical. J Phys Chem A 117:6629–6640
Funding
This work was financially supported by the Natural Science Foundations of China (No. 21707062) and the Scientific Research Starting Foundation of Mianyang Normal University (No. QD2016A007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Gerhard Lammel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 247 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., He, B., Sun, Y. et al. Theoretical investigations on mechanisms and pathways of CH2ClO2/CHCl2O2 with ClO reactions in the atmosphere. Environ Sci Pollut Res 27, 20457–20468 (2020). https://doi.org/10.1007/s11356-020-08315-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08315-0