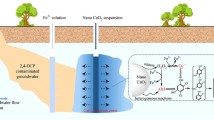
Abstract
The objectives of this study were to (1) conduct laboratory bench and column experiments to determine the oxidation kinetics and optimal operational parameters for trichloroethene (TCE)-contaminated groundwater remediation using potassium permanganate (KMnO4) as oxidant and (2) to conduct a pilot-scale study to assess the efficiency of TCE remediation by KMnO4 oxidation. The controlling factors in laboratory studies included soil oxidant demand (SOD), molar ratios of KMnO4 to TCE, KMnO4 decay rate, and molar ratios of Na2HPO4 to KMnO4 for manganese dioxide (MnO2) production control. Results show that a significant amount of KMnO4 was depleted when it was added in a soil/water system due to the existence of natural soil organic matters. The presence of natural organic material in soils can exert a significant oxidant demand thereby reducing the amount of KMnO4 available for the destruction of TCE as well as the overall oxidation rate of TCE. Supplement of higher concentrations of KMnO4 is required in the soil systems with high SOD values. Higher KMnO4 application resulted in more significant H+ and subsequent pH drop. The addition of Na2HPO4 could minimize the amount of produced MnO2 particles and prevent the clogging of soil pores, and TCE oxidation efficiency would not be affected by Na2HPO4. To obtain a complete TCE removal, the amount of KMnO4 used to oxidize TCE needs to be higher than the theoretical molar ratio of KMnO4 to TCE based on the stoichiometry equation. Relatively lower oxidation rates are obtained with lower initial TCE concentrations. The half-life of TCE decreased with increased KMnO4 concentrations. Results from the pilot-scale study indicate that a significant KMnO4 decay occurs after the injection due to the reaction of KMnO4 with soil organic matters, and thus, the amount of KMnO4, which could be transported from the injection point to the downgradient area, would be low. The effective influence zone of the KMnO4 oxidation was limited to the KMnO4 injection area (within a 3-m radius zone). Migration of KMnO4 to farther downgradient area was limited due to the reaction of KMnO4 to natural organic matters. To retain a higher TCE removal efficiency, continuous supplement of high concentrations of KMnO4 is required. The findings would be useful in designing an in situ field-scale ISCO system for TCE-contaminated groundwater remediation using KMnO4 as the oxidant.









Similar content being viewed by others
References
Al TA, Banks V, Loomer D, Parker BL, Ulrich Mayer K (2006) Metal mobility during in situ chemical oxidation of TCE by KMnO4. J Contam Hydrol 88(1):137–152
American Public Health Association (APHA) (2005) Standard Methods for the examination of water and wastewater, 22nd eden. APHA-AWWA-WEF, Washington, DC
Antoniou EA, Hartog N, van Breukelen BM, Stuyfzand PJ (2014) Aquifer pre-oxidation using permanganate to mitigate water quality deterioration during aquifer storage and recovery. Appl Geochem 50:25–36
Asghar BH, Fawzy A (2016) Kinetic, mechanistic, and spectroscopic studies of permanganate oxidation of azinylformamidines in acidic medium, with autocatalytic behavior of manganese(II). J Saudi Chemical Soc 20(5):561–569
Bennedsen, L.R. (2014) Chemistry of advanced environmental purification processes of water, pp. 13–74, Elsevier, Amsterdam
Chen J, Xu X, Pan X, Yao J, Li C, Qu R, Wang Z (2018) Mechanism insights into the oxidative degradation of decabromodiphenyl ethane by potassium permanganate in acidic conditions. Chem Eng J 332:267–276
Chen L, Liu Y, Liu F, Jin S (2014) Treatment of co-mingled benzene, toluene and TCE in groundwater. J Hazard Mater 275(Supplement C):116–120
Chowdhury AIA, Gerhard JI, Reynolds D, Sleep BE, O'Carroll DM (2017) Electrokinetic-enhanced permanganate delivery and remediation of contaminated low permeability porous media. Water Res 113:215–222
Cui J, Zhang L, Xi B, Zhang J, Mao X (2017) Chemical oxidation of benzene and trichloroethylene by a combination of peroxymonosulfate and permanganate linked by in-situ generated colloidal/amorphous MnO2. Chem Eng J 313:815–825
Dash S, Patel S, Mishra BK (2009) Oxidation by permanganate: synthetic and mechanistic aspects. Tetrahedron 65(4):707–739
Donne SW, Hollenkamp AF, Jonesa BC (2010) Structure, morphology and electrochemical behavior of manganese oxides prepared by controlled decomposition of permanganate. J Power Sources 195:367–373
Huang K-C, Hoag GE, Chheda P, Woody BA, Dobbs GM (2002) Kinetics and mechanism of oxidation of tetrachloroethylene with permanganate. Chemosphere 46(6):815–825
Huang Q, Dong H, Towne RM, Fischer TB, Schaefer CE (2014) Permanganate diffusion and reaction in sedimentary rocks. J Contam Hydrol 159:36–46
Hunkler D, Aravena R, Parker BL, Cherry JA, Diao X. (2003) Monitoring oxidation of chlorinated ethenes by permanganate in groundwater using stable isotopes: Laboratory and field studies. Environ Sci Technol 37:798–804
Jousse F, Atteia O, Höhener P, Cohen G (2017) Removal of NAPL from columns by oxidation, sparging, surfactant and thermal treatment. Chemosphere 188:182–189
Kao CM, Huang KD, Wang JY, Chen TY, Chien HY (2008) Application of potassium permanganate as an oxidant for in situ oxidation of trichloroethylene-contaminated groundwater: a laboratory and kinetics study. J Hazard Mater 153(3):919–927
Lee ES, Olson PR, Gupta N, Solpuker U, Schwartz FW, Kim Y (2014) Permanganate gel (PG) for groundwater remediation: compatibility, gelation, and release characteristics. Chemosphere 97(Supplement C):140–145
Li L, Wei D, Wei G, Du Y (2016) Oxidation of cefazolin by potassium permanganate: transformation products and plausible pathways. Chemosphere 149:279–285
Liang SH, Chen KF, Wu CS, Lin YH, Kao CM (2014) Development of KMnO4-releasing composites for in situ chemical oxidation of TCE-contaminated groundwater. Water Res 54:149–158
Loomer DB, Al TA, Banks VJ, Parker BL, Mayer KU (2011) Manganese and trace-metal mobility under reducing conditions following in situ oxidation of TCE by KMnO4: a laboratory column experiment. J Contam Hydrol 119(1–4):13–24
Mahmoodlu MG, Hassanizadeh SM, Hartog N (2014) Evaluation of the kinetic oxidation of aqueous volatile organic compounds by permanganate. Sci Total Environ 485–486:755–763
NIEA (National Institute of Environmental Analysis) (2008). Methods for groundwater sampling and hydrological tests, NIEA W103.54B, Taiwan Envoironmental Protection Administration, Taiwan
Rusevova K, Kopinke F-D, Georgi A (2012) Stabilization of potassium permanganate particles with manganese dioxide. Chemosphere 86(8):783–788
Shiau WJ (2007) Kinetic study of manganese dioxide formation in the permanganate oxidation of TCE in water. MS Thesis, Department of Environmental Engineering, National Cheng Kung University, Taiwan
SPSS, Stochastic Package for Social Science, 2007. Statistics Base 17.0 User’s Guide, ISBN-13-978-1-56827-400-3, https://www.jou.ufl.edu/assets/researchlab/SPSS-Statistcs- Base-Users-Guide-17.0.pdf
Tsai TT, Kao CM, Yeh TY, Liang SH, Chien HY (2009) Application of surfactant enhanced permanganate oxidation and biodegradation of trichloroethylene in groundwater. J Hazard Mater 161:111–119
Urynowicz MA, Balu B, Udayasankar U (2008) Kinetics of natural oxidant demand by permanganate in aquifer solid. J Contam Hydrol 96:187–194
West MR, Kueper BH (2012) Numerical simulation of DNAPL source zone remediation with in situ chemical oxidation (ISCO). Adv Water Resour 44:126–139
Yuan B, Chen Y, Fu M-L (2012) Degradation efficiencies and mechanisms of trichloroethylene (TCE) by controlled-release permanganate (CRP) oxidation. Chem Eng J 192:276–283
Yuan B, Li F, Chen Y, Fu M-L (2013) Laboratory-scale column study for remediation of TCE-contaminated aquifers using three-section controlled-release potassium permanganate barriers. J Environ Sci 25(5):971–977
Zhang S, Mao G, Crittenden J, Liu X, Du H (2017) Groundwater remediation from the past to the future: a bibliometric analysis. Water Res 119:114–125
Acknowledgements
We want to thank the personnel from the two sectors for their direction and help during the research period.
Funding
This study was funded in part by Taiwan Environmental Protection Administration and Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Yang, ZH., Ou, JH., Dong, CD. et al. Remediation of TCE-contaminated groundwater using KMnO4 oxidation: laboratory and field-scale studies. Environ Sci Pollut Res 26, 34027–34038 (2019). https://doi.org/10.1007/s11356-018-3099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3099-3