Abstract
Mercury is a toxic element. It undergoes biomagnification in the marine trophic chain, which is why it is significant to identify the factors influencing its bioaccumulation on the first level of the trophic chain. At present, the input of heavy metals to the southern Baltic is being reduced. On the other hand, the parameters influencing mercury remobilisation in the environment are a subject to a long-time trend associated with climate changes. Examples include growing number of heavy rain events causing surges or floods, and increased frequency of storm winds leading to increased coastal erosion as well as overall temperature increase. The present studies were carried out in the coastal zone of the Gulf of Gdańsk (southern Baltic) for 18 months at two stations (Chałupy and Osłonino) located in the Puck Lagoon, and for 12 months in Gdynia. Climate changes influence the abundance and species composition of phytoplankton, which in consequence has an effect on Hg accumulation and magnification in the trophic chain, and in the human body as a result. Extreme phenomena such as land erosion or floods resulted in an additional inflow of nutrients, but also toxic substances, into the coastal zone. The bioconcentration factor (BCF) increased almost four times after abrasion of cliff. That was conducive to the growth of microflora, as well as increased Hg accumulation. The highest bioconcentration of Hg in phytoplankton was observed when the Mesodinium rubrum (spring and autumn) and Diatomophyceae (winter) prevailed in biomass. The BCF was then almost tenfold higher than during the rest of the year.
Similar content being viewed by others
Introduction
Contemporary humans introduce into their bodies, whether consciously or not, a number of toxic substances including mercury (Hg). The latter is responsible for several ailments, for example causing neurological damage and kidney damage (Langford and Ferner 1999). The main route of Hg penetration into the human is the consumption of fish and seafood. Hence studies concerning the circulation of mercury in the marine environment and its introduction into the trophic chain are extremely significant.
The reduction in Hg emission contributed to a decrease in its inflow into the Baltic (HELCOM 2010), and has led to increased significance of point sources and the remobilisation of mercury from bottom sediments (Jędruch et al. 2015) and from land (Saniewska et al. 2014b). The latter processes are stimulated by intensive rains which lead to surges, and even floods. Climate change forecasts predict the occurrence of heavy rains (HELCOM 2013). Studies by Saniewska et al. (2014b) showed that the load of Hg introduced during one month of flooding on the Vistula river in 2010 was equivalent to 75% of the annual Hg load introduced with this river. At that time, the Hg concentration in the marine phytoplankton in the Vistula outlet area was four times higher than in the period before or after the flood (Saniewska et al. 2014b). Other extreme natural phenomena whose intensity is expected to heighten are storms and rising sea levels (HELCOM 2013), both of which result in increased coastal erosion and a consequent rise in mercury inflow into the Baltic (Bełdowska et al. 2016; Kwasigroch et al. 2018). It is particularly significant for organisms inhabiting the coastal zone, particularly in estuaries and semi-enclosed gulfs. The level of Hg concentration in the first link of the trophic chain, i.e. phytoplankton, also depended on the biomass of dominant species (Bełdowska and Kobos 2016).
Phytoplankton is a collection of microscopic plant organisms passively floating in the water column. In the Baltic Sea, like in many other seas, oceans and freshwater basins, seasonal changes are observed in phytoplankton’s structure. The growth and development of a given phytoplankton species depends on its ability to obtain necessary life resources and their apt utilisation at minimal loss. Seasonal changes in phytoplankton’s structure proceed in a characteristic way and are related to the ability of particular organisms to adapt to the changing environmental conditions. In the light of up-to-date studies, it can be said that in the yearly phytoplankton development cycle in the water of the southern Baltic, a sequence has been determined for the emergence of the representatives of particular taxons. The beginning of the vegetation period, in spring, is a time of mass diatom growth, later to be replaced by dinoflagellates. Thereafter, while dinoflagellates still constitute a large proportion, cyanophyta start to develop, reaching their peak development stage in summer. Autumn is a time of renewed diatom dominance, but with different species composition and in smaller numbers than in spring. Representatives of other taxons, with the exception of cryptophytes and the ciliate litostomate Mesodinium rubrum, occur in relatively small proportions (Pliński et al. 1982; Pliński and Picińska 1986; Pliński 1993; Niemkiewicz and Wrzołek 1998; Gromisz and Witek 2001; Witek 2010).
The reasons for anomalies in the composition, structure and abundance of phytoplankton may be the chemical fluctuation of water in the Baltic, caused mainly by anthropopressure and climate changes. Apart from increased water fertility, an important factor determining the dominance of particular species is temperature, the value of which fluctuates both seasonally and over periods of many years. At present, as a result of climate changes, the mean water temperature in a given month varies from year to year. In the coldest month—January—the mean air temperature varied within a range of 7.7 °C in the last few years (in 2010, − 5.5 °C; in 2015, 2.2 °C), while in the warmest month of the year—July—within a range of 3.3 °C (in 2010, 20.7 °C; in 2015, 17.4 °C) (IMGW PIB 2016). Reports by the Institute of Meteorology and Water Management National Research Institute (IMGW PIB 2016) indicate a rise in air temperature from year to year. Since 1998, winters have increasingly been classified as being above thermally normal: this happened 14 times prior to 2014, including 3 extremely warm and 4 anomal warm winters. While summers have been classified above thermally normal 15 times including 5 times extremely warm and 3 anomal warm. As a consequence, the biomass and species composition of phytoplankton change, and what follows is the inclusion of Hg into the trophic chain in the particular seasons in consecutive years.
The coastal zone of estuaries or small bays is particularly sensitive to temperature changes or an increase in the inflow of water pollutants. These are regions of intensive development, not only for planktonic organisms but also their consumers, including fish which are so attractive to humans as food. This point highlighted the need for studies to be carried out on the accumulation of mercury in phytoplankton in the particular months of the year in relation to the abundance and species composition of plankton, taking into account the forecast climate changes in the southern Baltic region. Methylmercury (MeHg) is the most toxic form for all organisms. This form is mainly biomagnified in aquatic food chains. The dominating form of Hg in seawater and in phytoplankton is inorganic mercury but Hg (II) absorbed by the organism can undergo endogenous methylation in gut by microbiome of animals in the lowest food webs like zooplankton. This process occurs in organisms at each level of the marine trophic web and can account for up to 70% of the annual MeHg uptake. (Wang and Wong 2003; Gorokhova et al. 2018). Therefore, analyses of total mercury concentration in phytoplankton are of great importance in the study of introducing Hg load to the marine trophic web. Phytoplankton and zooplankton are very important indicators for the biomagnification of Hg in fish and in consequence in human (Chen and Folt 2005).
Materials and methods
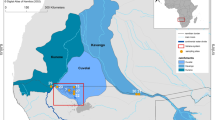
The studies were conducted in the southern Baltic region at three stations in the coastal zone of the Gulf of Gdansk: in Gdynia and in Osłonino, and Chałupy (Puck Lagoon). The Gdynia station was located in the open gulf, the Osłonino station was in the inner part of the gulf, enclosed on three sides and with a limited water exchange (Fig. 1). The Chałupy station was also in the inner part of the bay, but in an area with an increased water exchange with the open sea. The study samples were collected once a month, in the period: at the two stations in the Puck Lagoon between December 2011 and May 2013, and in Gdynia from December 2011 to November 2012. Due to icing on the coastal zone of the gulf, samples were not collected in February 2012, nor in Chałupy—December 2012 and January 2013—and in Osłonino—January, February, and March 2013. Phytoplankton was collected using a 20-μm net. The net was pulled several times just below the surface of the water to submerge the lower edge of the net to 0.5 m depth. This has caused concentration of collected plankton and subsequent clogging of the net. This has caused both the colonic picoplankton and the nanoplankton material occurring in surface and subsurface waters to be stopped by the clogged mesh. Phytoplankton samples were preserved at − 20 °C and were freeze-dried prior to analysis. For microscopic analyses, the samples were preserved with Lugol’s solution (1%), stored under dark and cool conditions.
Hg analyses
The mercury concentration was determined on the basis of analysis carried out on the thermo-desorption advanced mercury analyser AMA 254. Total Hg concentration was expressed in terms of dry weight (dw). The detection limit (included blank samples, replicates QA/QC) was 0.005 ng g −1. The measurements of mercury in the reference materials (BCR—414 (plankton)) were within the certified ranges, mean errors did not exceed 5%. Details of the analysis are described in Bełdowska and Kobos (2016).
Biological analyses
A quality and abundance analysis of phytoplankton was carried out using a reverse microscope (Nikon TMS, Tokio, Japan) equipped with phase contrast and 10×, 20×, 40× and 60× lenses. The non-densified material was left for 5–18 h for sedimentation in sedimentation chambers of 10 or 25 ml in capacity. Next, the material was analysed using Utermöhl’s method, according to the recommendations made by the Helsinki Commission (HELCOM 2008). Units were considered to be individual cells, cenobia, colonies and 100 μm trichome sections (ind. L−1). The volume and biomass of cells were calculated on the basis of geometric formulas according to the shapes of particular cells, and using size classes in accordance with the guidelines set out by Olenina et al. (2006).
Qualitative analysis of samples collected with plankton net and quantitative analysis of water samples did showed no significant mass of suspended sediments or other inorganic particles in the water column that could affect the measurements of plankton associated mercury.
Other parameters
The bioconcentration factor (BCF) was calculated the formula suggested by Szefer et al. (1999):
where Hgphytopl and Hgw represent concentrations of Hg in phytoplankton and seawater (Online Resource 1), respectively. To analyse mercury concertation, sweater was collected into acid-washed borosilicate vials with Teflon screw caps. Samples were oxidised by BrCl and pre-reduced with hydroxylamine hydrochloride solution. Analysis were conducted using cold vapour atomic fluorescence spectrometry CVAFS (TEKRAN 2600, Canada), according to US EPA method 1631 (US EPA 2002). Quality control procedures included blanks and water spiked with mercury nitrate in the range of 0.5–25 ng dm−3, and produced adequate precision (1% RSD) and recovery (98–99%). The detection limit was as low as 0.05 ng dm−3.
The characteristic of thermally condition was based on an excerpt from Climate Monitoring Bulletin, which is issued by the Institute of Meteorology and Water Management- National Research Institute (http://www.imgw.pl/extcont/biuletyn_monitoringu/). This classification was made on the basis of data collected during the years 1951–2010 which established the following categories: extremely cold, anomal cold, very cold, cold, lightly cold, thermally normal, lightly warm, warm, very warm, anomal warm, extremely warm (Online Resource 2).
Results and discussion
Hg concentrations in phytoplankton in the coastal zone of the Gulf of Gdansk range from 1.0 to 631.4 ng g−1 (Fig. 2). The mean Hg concentration amounted to 83 ng g−1, and the median was 51 ng g−1. The values depended both on the quantity and species composition of phytoplankton and on mercury sources in the gulf (Bełdowska and Kobos 2016). The load of mercury accumulated by phytoplankton can be influenced by the individual Hg sorption properties on the surface of cells of various phytoplankton groups. Studies into laboratory strains of diatoms, chlorophytes, cryptophytes and cyanophytes showed that phytoplankton cells accumulate Hg from natural water basins i.e. rivers and estuaries with the volume concentration factors of 0.4∙104–5.0∙104 (Pickhardt and Fisher 2007). The same studies showed that passive adsorption predominates over active absorption: about 85% of Hg bound to the cell wall and membranes, while only 15% of Hg was bound to the cytoplasm of diatom cells. However, direct Hg sorption by various microorganisms may be different. Pickhardt and Fisher (2007) showed that the volume concentration factor for cyanobacteria (145∙104) was an order of magnitude higher than that for eucaryotic phytoplankton (13∙104–64∙104), which can be explained by the greater cell surface: cell volume proportion in cyanobacteria than in the eucaryotic phytoplankton. On the other hand, Magulski et al. (2007) found that the cells of Nodularia spumigena cyanobacteria are capable of accumulating 28% of available reactive mercury forms, while diatoms (Cyclotella meneghiniana, Kützing) accumulate 65% of available mercury. There is a shortage of laboratory reports on the ability of the ciliate Mesodinium rubrum to absorb this material.
Concentration of Hg (ng g−1 dw in phytoplankton at Chałupy, Osłonino (December 2011 to May 2013) and Gdynia (December 2011 to November 2012) stations. Data form Chałupy and Oslonino stations based on Bełdowska and Kobos (2016)
Hg bioconcentration factor (logBCF) in phytoplankton from Puck Bay ranged from 0.02∙104 to 28.0∙104 (logBCF 2.3–5.4) (Table 1) which is comparable to phytoplankton from Long Island Sound: logBCF 2.6–5.5 (Gosnell et al. 2017). The mean BCF for all study period was 4.0∙104 (logBCF 4.2). The lowest mean bioaccumulation was during summer period 1.4∙104 (mean logBCF 3.8), when the biomass of phytoplankton groups was the highest, which is probably due to the biodilution of Hg. During winter, the bioaccumulation process was the highest: mean 5.3∙104 (mean logBCF 4.5). Mesodinum rubrum was the organisms which most effectively accumulated mercury from water: April 2012 BCF = 28∙104 (logBCF 5.4) and October 2012: 17∙104 (logBCF 5.2). Also high BCF was when Diatomophyceae dominated: December 2011 (mainly Pennales) 14∙104 (logBCF 5.2) and January 2012 (mainly Synedra ulna) 9.6104 (logBCF 5.0).
The seasonal variability of Hg concentration
Spring
In thermal classification, 70% of springs between 1987 and 2016 were anomal warm (IMGW PIB 2016). As a result of a rise in water temperature in springtime (March, April, May), no blooms were observed of the spring diatoms Skeletonema marinoi (formerly S. costatum) or Chateroceros spp., Pauliella (Achnanthes) taeniata, Thalassiosira levanderii or the dinoflagellates Heterocapsa triquetra (Fig. 3). These species had been commonly observed in the study area in the previous years (Pliński 1993; Witek et al. 1993; Dobroń 2004). The characteristic peak in the growth of diatom biomass was not observed either.
Biomass of phytoplankton groups (mg dm−3) changes in Chałupy and Osłonino (December–May 2013) and Gdynia (December 2011–November 2012). The OY scale is logarithmic. Data form Chałupy and Oslonino stations based on Bełdowska and Kobos (2016)
In the first months after the icing of the gulf, Hg concentrations in phytoplankton were similar to conditions just before the freezing (Fig. 2; Online Resource 2). A further increase in water temperature and solar radiation in April 2012 was conducive to the emergence of M. rubrum, which has got high potential for mercury accumulation (Bełdowska and Kobos 2016) (Fig. 3). As a consequence, Hg concentrations in phytoplankton at Osłonino rose ninefold compared to March (Fig. 2). The presence of Ciliata in May in region of Gdynia led to increase of Hg concentration, too. At Chałupy, the increase in Hg concentration in phytoplankton was smaller, probably owing to the presence of dinoflagellates (20% of mass), which accumulate less Hg. In 2013, the icing on the gulf persisted for a longer period of time than in 2012 and this was probably the reason why fewer litostomate and dinoflagellates survived the low temperatures. As a consequence, diatoms were found to be predominant in spring at both stations. At Osłonino, where in 2013 the icing persisted till the end of March, M. rubrum constituted a small percentage of biomass, which resulted in Hg concentration in phytoplankton in April 2013 being much lower than in April 2012 (Fig. 2). Near Chałupy the coastal zone was not iced as in February 2013. In that case, low air temperature, causing greater mercury atmospheric deposition (both wet and dry) (Bełdowska et al. 2014; Saniewska et al. 2014a), was conducive to the increase of Hg concentration in phytoplankton (Bełdowska and Kobos 2016). This process is of particular importance in shallow areas, where solar radiation accelerates the melting of ice, and water warming with simultaneous elevated nutrient concentration, causes algae growth. Such conditions were so favourable for the growth of phytoplankton that the numbers of phytoplankton were nearly as high as in August, when the highest numbers were observed (Fig. 3). In April 2013, when the increase in air temperature reduced the need to heat buildings by fossil fuel combustion, leading to a consequent decrease in the atmospheric input of Hg, Hg concentration in phytoplankton dropped compared to March.
Summer
The summer months (May to August) of the years 1987–2015 were also classified mostly as thermally anomal (IMGW PIB 2016). In the study period, the typical planktonic bloom of cyanobacteria belongs to the genera Nodularia, Aphanizomenon and Dolichospermum were not observed in the Puck Lagoon. Only at the station in Gdynia there were numerous occurrence of Cyanophyceae (Fig. 3). These organisms create blooms mainly in July and August in the waters of the Gulf of Gdansk and the entire Baltic Sea (Mazur-Marzec et al. 2006; Mazur-Marzec and Pliński 2009; Suikkanen et al. 2010). Hg concentrations in phytoplankton at all stations were at their lowest (Fig. 2). Even in the months when the dominant species was Mesodinum rubrum (May 2012 in Chałupy, July 2012 in Osłonino), which intensively accumulated Hg in autumn and early spring, no increase in mercury concentration was found in phytoplankton. This, on the one hand, was caused by a smaller dry deposition of Hg (Bełdowska et al. 2014), and on the other, by a large rise in biomass which led to the biodilution of Hg in phytoplankton (Mason et al. 1996; Luengen and Flegal 2009; Hammerschmidt et al. 2013). Another factor influencing the drop in Hg in phytoplankton was the detoxification of phytoplankton from mercury under the influence of solar radiation (Costa and Liss 2000).
On the other hand, high water temperatures in summer are favourable (Online Resource 2) for intense phytoplankton growth and in the consequence Hg concentration in alga biomass increase, despite relatively low Hg concentration (Bełdowska and Kobos 2016). If there is an increased input of metal during this period, for example with wet precipitation, particularly during the movement of continental air masses (Bełdowska et al. 2014) and in run-off water from urbanised areas (Saniewska et al. 2014c), this can additionally increase the metal load in phytoplankton biomass. Climate change forecasts for the southern Baltic predict an increase of precipitation during the summer (HELCOM 2013), which may also contribute to a rise in the Hg load introduced to the trophic chain during the summer season (Saniewska et al. 2014a).
Autumn
Autumn time encompasses September, October and November. Between 1997 and 2016, there have been 15 exceedances of normal temperature within this season (IMGW PIB 2016). In autumn 2012, the typical cryophilic planktonic diatoms of the Skeletonema marinoi and Pauliella (Achnanthes) taeniata types constituted only a small percentage of total phytoplankton biomass. At that time, the characteristic bloom of large centric diatoms Coscinodiscus granii was not observed (Fig. 3). This species has often been indicated to be a typical autumn dominant (Pliński 1993; Dobroń 2004; Witek 2010). The peak of domination for autumn diatoms was much more varied that in spring, which is related to the warming of the autumn season in multinannual terms.
In the study period (2012), September was classified as a very warm month (Online Resource 2). This resulted in water temperature (15.6–17.8 °C) being on a similar level to that of August (16.8–20.1 °C), and such conditions were conducive to the growth of phytoplankton. The numbers of phytoplankton at the Gdynia station in that month were as much as six times higher than in the previous month, while near Chałupy, the algae biomass was 10% larger than in August. The increase in the proportion of diatoms and cyanobacteria at Chałupy was favourable for better Hg adsorption from the water, the consequence of that being a twofold increase of Hg concentration in phytoplankton compared with August (Figs. 2 and 3). At the two remaining stations, dinoflagellates were predominant in terms of mass, which led to a drop in Hg in phytoplankton down to a few ng g−1 dw (Bełdowska and Kobos 2016). The predominance of this alga species in autumn has a hindering effect on Hg introduction to the trophic chain.
November 2012 was classed as thermally normal (Online Resource 2). The numbers of phytoplankton dropped at all stations, but water temperature (10.2–13.0 °C) was high enough for large algae such as M. rubrum to grow at Chałupy (53%) and Osłonino (89%) (Bełdowska and Kobos 2016) and for diatoms to thrive at Gdynia (91%) (Fig. 3). In that period, air temperature was low enough for buildings to be heated by fossil fuel combustion; hence, atmospheric deposition was an additional source of the labile Hg form in water (Bełdowska and Kobos 2016). This factor, combined with the presence of M. rubrum, which intensively accumulated Hg, resulted in the highest concentration of this metal beinng determined in algae both at Chałupy and at Osłonino (Fig. 3).
In the following month of the autumn season, November, air temperature was high enough for this month to be classified as very warm (Online Resource 2). Mean water temperature at that time ranged from 8.6 to 9 °C. These conditions were conducive to maintaining the same numbers and biomass of algae as in October at Chałupy, and to exceeding those values at Osłonino (Fig. 3). At Osłonino, despite the rise in numbers (43-fold) and alga biomass (by 43%), Hg concentration in phytoplankton dropped, probably as result of the change of dominant from M. rubrum (in October) to diatoms (Fig. 2). At Chałupy, as at Osłonino, diatoms were predominant at that time, but the 13% proportion of M. rubrum was enough for Hg levels in phytoplankton cells to be close to those observed in October (Fig. 2).
An additional source of Hg in phytoplankton in November at Gdynia was the rubble released during the controlled removal of a past-war bunker, which was knocked off the top of the cliff (www.trojmiasto.pl). That took place seven days before the samples were collected. As a consequence, a large amount of sedimentary material was introduced into the water along with the bunker. The mean Hg concentration in the cliff in this area was 9 ng g−1 dw (Bełdowska et al. 2016), but the sediment mass was large enough to cause a 10-fold increase of Hg concentration (496.3 ng g−1) in phytoplankton (Fig. 2), and a 2.5-fold increase in suspended matter (Bełdowska 2015). The bioconcentration factor of mercury in phytoplankton was almost four times higher than mean value in this region (Table 1). In the southern Baltic, the sea level has been rising in recent decades (Harf et al. 2001; Johansson et al. 2004), resulting in the elution of land with accumulated pollutants. The data presented here suggest that this has a significant influence on the size of Hg load introduced to the first level of trophic chain.
Winter
In the southern Baltic, in terms of climate changes, there is a tendency towards the winter season (December, January, February) becoming warmer and this either prevents sea water from freezing, or shortens the period of time for which it is frozen (IMGW PIB 2016). Between 1946 and 1991, the average icing period of the Puck Lagoon was 90 days, while for the Gulf of Gdansk, close to Gdynia, it was 30 days (Girjatowicz and Kożuchowski 1999; Szefler 1993). At that time, ice appeared in the Puck Lagoon on 19 December and disappeared on 18 March, while in the outer part of the bay, close to the Gdynia station, icing appeared on 21 January and melted away by 4 March. During this time the numbers of plankton were very limited. In 2012, the period of icing on the bay was much shorter, lasting for 30 days (28 January–26 February). Warmer winters have resulted in the lack of dominants such as Pauliella (Achnanthes) taeniata and Thalassiosira levanderii (Fig. 3), species which were known to thrive and dominate in winter phytoplankton of the Puck Lagoon (Pliński 1993), and the Gulf of Gdansk (Witek et al. 1997; Dobroń 2004).
December 2011 was an extremely warm month (Online Resource 2), but low temperatures in November had brought water temperature down to 1.9–4.2 °C. At that time, particularly in Chałupy and Gdynia where water mixing was stronger, the alga biomass was small (3.5 μg L−1 and 3.9 μg L−1 respectively) but the predominance of diatoms resulted in a raised Hg concentration (Figs. 2 and 3). This is most probably related to the fact that Hg from fossil fuel combustion is easily adsorbed (Bełdowska and Kobos 2016). December 2012 was thermally classed as being only lightly warm (IMGW PIB 2016) but that was determined mainly by the last few days before the samples were collected, during which air temperature dropped considerably. At that time, the bay at Chałupy was iced over. This simultaneously contributed to a raised introduction of atmospheric Hg into water at Osłonino. As water temperature drops much more slowly than that of air, and November 2012 was very warm (IMGW PIB 2016), the numbers, biomass and dominant (diatoms) of phytoplankton in December 2012 were similar to those of the preceding month. At the same time, the numbers and biomass recorded for the lightly warm December 2012 were respectively 10 and 15 times higher than in the extremely warm December 2011. This shows that a warm winter following a warm autumn is conducive to raised Hg levels persisting over time, and influences phytoplankton biomass, which increases the load of toxic mercury in the first link of the trophic chain.
In January 2012, samples were collected on the 16th, and up to that day, the month was thermally classed as being very warm, demonstrating water temperature (particularly in Osłonino and Chałupy) similar to that of the preceding month (1.7–2.8 °C) (Online Resource 2). At that time, Hg concentration in phytoplankton was slightly lower than in the preceding month, with the exception of Gdynia, where no phytoplankton was found (Fig. 2). Seeing as the biomass and numbers of algae were comparable to those in the preceding month, and at Chałupy even higher, the influence was probably that of the species composition of phytoplankton: at Chałupy a smaller proportion of Mesodinum rubrum in biomass (drop from 13 to 3%), which effectively accumulates Hg. At Osłonino, on the other hand, an increase from 5 to 22% in the proportion of dinoflagellates, which are the least effective at Hg accumulation among the analysed phytoplankton species (Fig. 3).
In February 2012 at all stations and in February 2013 at Osłonino, the waters of the gulf were iced over. In February 2013, at Chałupy, the shallow depth and greater water dynamics, in comparison with Osłonino, were conducive to the faster melting of the ice. Sunny days resulted in a water temperature of 5 °C in the shallow coastal zone (depth of 0.5 m on the day of collection). Such conditions were favourable for the development of cryophilic diatoms (78%), while low air temperatures (around 0 °C) were conducive to increased Hg emission from fossil fuel combustion and its deposition into the gulf. This in turn contributed to the faster inclusion of Hg into the trophic chain (Fig. 2).
Conclusion
Seasonal changes in phytoplankton’s structure proceed in a characteristic way and are related to the ability of the particular organism groups to adapt to the changing environmental conditions. In the water of the southern Baltic, in spring, there is a massive growth of diatoms which are later replaced by dinoflagellates. Later, while dinoflagellates still constitute a large share, cyanobacteria start to develop, reaching their development peak in summer. In autumn, diatoms prevail once again but the species composition is different and the quantities are smaller than in spring. Representatives of other taxons, with the exception of cryptophytes and the ciliate Mesodinium rubrum, occur in relatively small proportions. The reason for the aberrances in the composition and quantity of phytoplankton may be fluctuations in water chemistry, as well as climate changes.
Climate changes also manifest themselves in the intensification of extreme natural phenomena, such as floods, increased average wave height and strong winds (HELCOM 2013), which contribute to the erosion of the southern Baltic coastline. As a consequence, mercury deposited in terrestrial reservoirs during many years could introduce to the sea in single strong pulse (Bełdowska et al. 2016) which could cause an increase of Hg bioconcentration in phytoplankton—the first link of the trophic chain.
References
Bełdowska M (2015) The influence of weather anomalies on mercury cycling in the marine coastal zone of the southern Baltic—future perspective. Water Air Soil Pollut 226:2248. https://doi.org/10.1007/s11270-014-2248-7
Bełdowska M, Kobos J (2016) Mercury concentration in phytoplankton in response to warming of an autumn - winter season. Environ Pollut 215:38–47. https://doi.org/10.1016/j.envpol.2016.05.002
Bełdowska M, Saniewska D, Falkowska L (2014) Factors influencing variability of mercury input to the southern Baltic Sea. Mar Pollut Bull 86:283–290. https://doi.org/10.1016/j.marpolbul.2014.07.004
Bełdowska M, Jędruch A, Łęczyński L, Saniewska D, Kwasigroch U (2016) Coastal erosion as a source of mercury into the marine environment along the Polish Baltic shore. Environ Sci Pollut Res 23(16):16372–16382. https://doi.org/10.1007/s11356-016-6753-7
Chen CY, Folt CL (2005) High plankton densities reduce mercury biomagnification. Environ Sci Technol 39(1):115–121
Costa M, Liss P (2000) Photoreduction and evaluation of mercury from seawater. Sci Total Environ 261:125–135
Dobroń K (2004) Long- and short-term changes in the structure of the phytoplankton of the Gulf of Gdansk with particular emphasis on the role of dinoflagellates. Monography, Institute of Oceanography, Library of the University of Gdańsk (dissertation in Polish), p 147
Girjatowicz JP, Kożuchowski K (1999) Variations of thermic and ice conditions in the Szczecin lagoon region. In: Jarvet A (ed) Publ 2nd workshop on the Baltic Sea ice climate. Publicationes Instituti Geographici Universitatis Tartuensis vol 84, Tartu, pp 69–73
Gorokhova E, Soerensen AL, Motwani NH (2018) Mercury-methylating bacteria are associated with zooplankton: a proof-of-principle survey in the Baltic Sea. BioRxiv. https://doi.org/10.1101/279976
Gromisz S, Witek Z (2001) Main phytoplankton assemblages in the Gulf of Gdańsk and the Pomeranian Bay from 1994 to 1997. Bull Sea Fish Inst 153:31–51
Hammerschmidt CR, Finiguerra MB, Weller RL, Fitzgerald WF (2013) Methylmercury accumulation in plankton on the continental margin of the Northwest Atlantic Ocean. Environ Sci Technol 47:3671–3677
Harf J, Frischbutter A, Lampe R, Meyer M (2001) Sea level changes in the Baltic Sea. - Interrelation of Climatic and Geological Processes. In: Gerhard LC, Harrison WE, Hanson BM (eds) Geological perspectives of global climate change. AAPG-Studies in Geology 47:231–250
HELCOM. (2008). Manual for Marine Monitoring in the COMBINE Program (Annex C-6) http://helcom.fi/Documents/Action%20areas/Monitoring%20and%20assessment/Manuals%20and%20Guidelines/Manual%20for%20Marine%20Monitoring%20in%20the%20COMBINE%20Programme%20of%20HELCOM.pdf
HELCOM (2010) Towards a tool for quantifying anthropogenic pressures and potential impacts on the Baltic Sea marine environment: a background document on the method, data and testing of the Baltic Sea pressure and impact indices. Helsinki Commission, Baltic Sea environmental proceedings no. 125, p 72. https://doi.org/10.13140/RG.2.1.2148.6961
HELCOM (2013) Climate change in the Baltic Sea area: HELCOM thematic assessment in 2013. Helsinki Commission, Baltic Sea Environment Proceedings No. 137, Helsinki, p 70
IMGW PIB (Institute of Meteorology and Water Management National Research Institute). 2016. Polish Climate Monitoring Bulletin. http://www.imgw.pl/extcont/biuletyn_monitoringu
Jędruch A, Bełdowski J, Bełdowska M (2015) Long-term changes and distribution of mercury concentrations in surface sediments of the Gdańsk Basin (southern Baltic Sea). J Soils Sediments 15:2487–2497. https://doi.org/10.1007/s11368-015-1148-9
Johansson MM, Kahma KK, Borman H, Launiainen J (2004) Scenarios for sea level rise on the Finnish coast. Boreal Environ Res 9:153–166
Langford N, Ferner R (1999) Toxicity of mercury. J Hum Hypertens 13:651–656
Kwasigroch U, Bełdowska M, Jędruch A, Saniewska D (2018) Coastal erosion—a "new" land-based source of labile mercury to the marine environment. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-018-2856-7
Luengen AC, Flegal AR (2009) Role of phytoplankton in mercury cycling in the San Francisco Bay estuary. Limnol Oceanogr 54(1):23–40
Magulski R, Falkowska L, Bełdowska M (2007) Mercury transformations in the seawater in the presence of cyclotella meneghiniana and nodularia spumigena. Oceanol Hydrobiol Stud 36:69–81
Mason RP, Reinfelder JR, Morel FMM (1996) Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ Sci Technol 30:1835–1845
Mazur-Marzec H, Pliński M (2009) Do toxic cyanobacteria blooms pose a threat to the Baltic ecosystem. Oceanologia 51(3):293–319
Mazur-Marzec H, Krężel A, Kobos J, Pliński M (2006) Toxic Nodularia spumigena blooms in the coastal waters of the Gulf of Gdańsk: a ten-year survey. Oceanologia 48(2):255–273
Niemkiewicz E, Wrzołek L (1998) Phytoplankton as eutrophication indicator in the Gulf of Gdańsk water. Oceanol Stud 4:77–92
Olenina I, Hajdu S, Andersson A, Edler L, Wasmund N, Busch S, Göbel J, Gromisz S, Huseby S, Huttunen M, Jaanus A, Kokkonen P, Ledaine I, Niemkiewicz E (2006) Biovolumes and size-classes of phytoplankton in the Baltic Sea. Balt Sea Environ Proc (Helsinki commission, Helsinki) 106:1–144
Pickhardt PC, Fisher NS (2007) Accumulation of inorganic and monomethylmercury by freshwater phytoplankton in two contrasting water bodies. Environ Sci Technol 41:125–131
Pliński M (1993) Phytoplankton. In: Korzeniewski K (ed) Puck Bay. Instytut Oceanografii Uniwersytetu Gdańskiego, Gdańsk, pp 378–387 (in Polish)
Pliński M, Picińska J (1986) The dynamics of seasonal change of the phytoplankton biomass in the Gulf of Gdańsk. Oceanologia 23:77–83
Pliński M, Sobolewska B, Mielczarek M (1982) The composition and abundance of phytoplankton western part of the Gulf of Gdańsk. Stud Mater Oceanol 39:34–75 (in Polish)
Saniewska D, Bełdowska M, Bełdowski J, Falkowska L (2014a) Mercury in precipitation at urbanized coastal zone of Baltic (Poland). AMBIO 43:871–877
Saniewska D, Bełdowska M, Bełdowski J, Jędruch A, Saniewski M, Falkowska L (2014b) Mercury loads into the sea associated with extreme flood. Environ Pollut 2014b 191:93–100
Saniewska D, Bełdowska M, Bełdowski J, Saniewski M, Szubska M, Romanowski A, Falkowska L (2014c) The impact of land use and season on the riverine transport of mercury into the marine coastal zone. Environ Monit Assess 186(11):7593–7604
Suikkanen S, Kaartokallio H, Hällfors S, Huttunen M, Laamanen M (2010) Life cycle strategies of bloom-forming, filamentous cyanobacteria in the Baltic Sea. Deep-Sea Res II 57:199–209
Szefer P, Ali AA, Ba-Haroon AA, Rajeh AA, Geldon L, Nabrzyski M (1999) Distribution and relationships of selected trace metals in molluscs and associated sediments from the Gulf of Aden, Yemen. Environ Pollut 106:299–314. https://doi.org/10.1016/S0269-7491(99)00108-6
Szefler K (1993) Ice cover. In: Korzeniewski K (ed) Puck Bay. Fundacja Rozwoju Uniwerystetu Gdańskiego, Gdańsk, 112–134 (in Polish)
US EPA (US Environmental Protection Agency) (2002) Method 1631, Revision E: Mercury in water by oxidation, purge and trap, and cold vapour atomic fluorescence spectrometry. In: US Environmental Protection Agency, Office of Water 4303, Washington
Wang W-X, Wong RSK (2003) Bioaccumulation kinetics and exposure pathways of inorganic mercury and methylmercury in a marine fish, the sweetlips Plectorhinchus gibbosus. Mar Ecol Prog Ser 261:257–268
Witek B (2010) Short-term fluctuations of phytoplankton in the coastal zone of the Gulf of Gdańsk. Wydawnictwo Uniwersytetu Gdańskiego, Gdańsk 108 pp. (in Polish)
Witek Z, Bralewska J, Chmielewski H, Drgas A, Gostkowska J, Kopacz M, Knurowski J, Krajewska-Sołtys J, Lorenz Z, Maciejewska K, Mackiewicz T, Nakonieczny J, Ochocki S, Warzocha J, Piechura J, Renk H, Stopiński M, Witek B (1993) Structure and function of marine ecosystem in the Gdańsk Basin on the basis of studies performed in 1987. Stud Mater Oceanol 63:5–125
Witek Z, Ochockil S, Maciejowska M, Pastuszakl M, Nakonieczny J, Podgorska B, Kownacka JM, Mackiewicz T, Wrzesinska-Kwiecień (1997) Pytoplankton primary production and its utilization by the pelagic community in the coastal zone of the Gulf of Gdańsk (southern Baltic). Mar Ecol Prog Ser 148:169–186
Acknowledgments
This study has been performed within the framework of a National Science Centre project No. 2011/01/B/ST10/07697.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bełdowska, M., Kobos, J. The variability of Hg concentration and composition of marine phytoplankton. Environ Sci Pollut Res 25, 30366–30374 (2018). https://doi.org/10.1007/s11356-018-2948-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2948-4