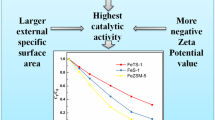
Abstract
Environmentally emerging micro-pollutant, caffeine, was mineralized (i.e., full degradation) by the isomorphic incorporation of Fe into silicalite-1 (mordenite framework inverted (MFI) structure zeolite) through a microwave synthesis method. The Fe incorporation conferred mesopore formation that facilitated caffeine access and transport to the MFI zeolite structure. Increasing the Fe content favored the formation of Fe(O)4 sites within the MFI structure. The catalytic activity for the degradation of caffeine increased as a function of Fe(O)4 sites via a Fenton-like heterogeneous reaction, otherwise not attainable using Fe-free pure MFI zeolites. Caffeine degradation reached 96% (TOC based) for zeolites containing 2.33% of Fe.









Similar content being viewed by others
References
Anizelli PR, Baú JPT, Valezi DF, Canton LC, Carneiro CEA, Di Mauro E, da Costa ACS, Galante D, Braga AH, Rodrigues F, Coronas J, Casado-Coterillo C, Zaia CTBV, Zaia DAM (2016) Adenine interaction with and adsorption on Fe-ZSM-5 zeolites: a prebiotic chemistry study using different techniques. Microporous and Mesoporous Mater 226:493–504
An W, Xiao X, Yu M, Chen X, Xu Y, Zhou W (2013) Adsorptive removal of trace oxytetracycline from water by acid-modified zeolite: influencing factors. Water Sci Technol 68:2473–2478
Banerjee S, Verma P, Mitra R, Basu G, Pal S (2012) Probing the interior of self-assembled caffeine dimer at various temperatures. J Fluoresc 22:753–769
Bordiga S, Buzzoni R, Geobaldo F, Lamberti C, Giamello E, Zecchina A, Leofanti G, Petrini G, Tozzola G, Vlaic G (1996) Structure and reactivity of framework and extraframework iron in Fe-silicalite as investigated by spectroscopic and physicochemical methods. J Catal 158(2):486–501
Bruton T, Alboloushi A, de la Garza B, Kim BO, Halden RU (2010) Fate of caffeine in the environment and ecotoxicological considerations. J Am Chem Soc 1048:257–273
Buerge IJ, Poiger T, Müller MD, Buser H-R (2003) Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ Sci Technol 37:691–700
Christensen CH, Johannsen K, Schmidt I, Christensen CH (2003) Catalytic benzene alkylation over mesoporous zeolite single crystals: improving activity and selectivity with a new family of porous materials. J Am Chem Soc 125(44):13370–13371
Fan F, Feng Z, Li C (2010) UV Raman spectroscopic study on the synthesis mechanism and assembly of molecular sieves. Chem Soc Rev 39:4794–4801
Fan F, Sun K, Feng Z, Xia H, Han B, Lian Y, Ying P, Li C (2009) From molecular fragments to crystals: a UV Raman spectroscopic study on the mechanism of Fe-ZSM-5 synthesis. Chem A Eur J 15:3268–3276
Franzoso F, Nisticò R, Cesano F, Corazzari I, Turci F, Scarano D, Prevot AB, Magnacca G, Carlos L, Mártire DO (2017) Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem Eng J 310:307–316
Gao Y, Wang Y, Zhang H (2015) Removal of rhodamine B with Fe-supported bentonite as heterogeneous photo-Fenton catalyst under visible irradiation. Appl Cat Environ 178:29–36
Giordano G, Katovic A, Perathoner S, Pino F, Centi G, Nagy JB, Lazar K, Fejes P (2002) One-step benzene oxidation to phenol—part I: preparation and characterization of Fe-(Al)MFI type catalysts. Stud Surf Sci Catal 142:477–484
Gonzalez-Olmos R, Holzer F, Kopinke F-D, Georgi A (2011) Indications of the reactive species in a heterogeneous Fenton-like reaction using Fe-containing zeolites. App Catal Gen 398:44–53
Gummadi SN, Ganesh KB, Santhosh D (2009) Enhanced degradation of caffeine by immobilized cells of Pseudomonas sp. in agar-agar matrix using statistical approach. Biochem Eng J 44:136–141
Hoffmann K, Marlow F, Caro J (1997) Photoinduced switching in nanocomposites of azobenzene and molecular sieves. Adv Mater 9(7):567–570
Jung J, Jo C, Mota FM, Cho J, Ryoo R (2015) Acid catalytic function of mesopore walls generated by MFI zeolite desilication in comparison with external surfaces of MFI zeolite nanosheet. Appl Catal A Gen 492:68–75
Klamerth N, Malato S, Agüera A, Fernández-Alba A, Mailhot G (2012) Treatment of municipal wastewater treatment plant effluents with modified photo-Fenton as a tertiary treatment for the degradation of micro pollutants and disinfection. Environ Sci Technol 46:2885–2289
Kragović M, Daković A, Marković M, Krstić J, Gatta GD, Rotiroti N (2013) Characterization of lead sorption by the natural and Fe (III)-modified zeolite. Appl Surf Sci 283:764–774
Kritchayanon N, Thanabodeekij N, Jitkarnka S, Jamieson AM, Wongkasemjit S (2006) Synthesis, of Fe-loaded MFI zeolite using silatrane as precursor and its CO activity. Appl Organomet Chem 20:155–160
Li JPH, Kennedy E, Stockenhuber M (2014) Oxidative coupling and hydroxylation of phenol over transition metal and acidic zeolites: insights into catalyst function. Catal Lett 144:9–15
Li X, Li B, Xu J (2013) Synthesis and characterization of transitional metal-rich zeolite M-MFI (M=Fe, co, Ni, cu) with regular mesoporous channels. Coll Surf A Physicochem Eng Asp 434:287–295
Li Y-S, Church JS, Woodhead AL (2012) Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J Magn Magn Mater 324:1543–1550
Liu C, Li J, Qi J, Wang J, Luo R, Shen J, Sun X, Han W, Wang L (2014) Yolk-shell Fe(0)@SiO2 nanoparticles as nanoreactors for Fenton-like catalytic reaction. ACS Appl Mater Interfaces 6:13167–13173
Maxwell IE, van den Brink P, Downing RS, Sijpkes AH, Gomez S, Maschmeyer T (2003) High-throughput technologies to enhance innovation in catalysis. Top Catal 24:125–135
Meng Q, Doetschman DC, Rizos AK, Lee M-H, Schulte JT, Spyros A, Kanyi CW (2011) Adsorption of organophosphates into microporous and mesoporous NaX zeolites and subsequent chemistry. Environ Sci Technol 45(7):3000–3005
Mijangos F, Varona F, Villota N (2006) Changes in solution color during phenol oxidation by Fenton reagent. Environ Sci Technol 40:5538–5543
Moliner M (2012) Direct synthesis of functional zeolitic materials. ISRN Mater Sci 2012. https://doi.org/10.5402/2012/789525
Pérez-Ramírez J, Christensen CH, Egeblad K, Christensen CH, Groen JC (2008) Hierarchical zeolites: enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem Soc Rev 37(11):2530–2542
Rangnekar N, Mittal N, Elyassi B, Caro J, Tsapatsis M (2015) Zeolite membranes—a review and comparison with MOFs. Chem Soc Rev 44(20):7128–7154
Rodriguez del Rey Z, Granek EF, Sylvester S (2012) Occurrence and concentration of caffeine in Oregon coastal waters. Mar Pollut Bull 64:1417–1424
Rodríguez S, Santos A, Romero A (2017) Oxidation and priority and emerging pollutants with persulfate activated by iron: effect of iron valence and particle size. Chem Eng J 318:197–205
Rosal R, Rodríguez A, Perdigón-Melón JA, Petre A, García-Calvo E, Gómez MJ, Agüera A, Fernández-Alba AR (2009) Degradation of caffeine and identification of the transformation products generated by ozonation. Chemosphere 74:825–831
Taniguchi T, Nakasaka Y, Yoneta K, Tago T, Masuda T (2016) Size-controlled synthesis of MFI metallosilicate and their catalytic performance on acetone to olefins reaction. Microporous Mesoporous Mater 224:68–74
Treacy MMJ, Higgins JB (2001) Collection of simulated XRD powder patterns for zeolites, 4th edn. Elsevier, Amsterdam
Vermeiren W, Gilson JP (2009) Impact of zeolites on the petroleum and petrochemical industry. Top Catal 52(9):1131–1161
Wang Y, Zhao G, Chai S, Zhao H, Wang Y (2013) Three-dimensional homogeneous ferrite-carbon aerogel: one pot fabrication and enhanced electro-Fenton reactivity. ACS Appl Mater Interfaces 5:842–852
Wingenfelder U, Hansen C, Furrer G, Schulin R (2005) Removal of heavy metals from mine waters by natural zeolites. Environ Sci Technol 39(12):4606–4613
Zeng T, Zhang X, Wang S, Niu H, Cai Y (2015) Spatial confinement of a Co3O4 catalyst in hollow metal–organic frameworks as a nanoreactor for improved degradation of organic pollutants. Environ Sci Technol 49(4):2350–2357
Zubir NA, Yacou C, Motuzas J, Zhang X, Diniz da Costa JC (2014) Structural and functional investigation of graphene oxide-Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci Rep 4:4594. https://doi.org/10.1038/srep04594.
Zubir NA, Yacou C, Motuzas J, Zhang X, Zhao XS, Diniz da Costa JC (2015) The sacrificial role of graphene oxide in stabilising a Fenton-like catalyst GO–Fe3O4. Chem Commun 51:9291–9293
Acknowledgments
The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland. A. Julbe and J.C. Diniz da Costa would like to acknowledge the financial support for international collaboration from the Centre National de la Recherche Scientifique (CNRS-INC) in France. J. C. Diniz da Costa acknowledges support given by the Australian Research Council (ARC) Future Fellowship program (FT130100405).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Motuzas, J., Drobek, M., Martens, D.L. et al. Environmental mineralization of caffeine micro-pollutant by Fe-MFI zeolites. Environ Sci Pollut Res 25, 3628–3635 (2018). https://doi.org/10.1007/s11356-017-0530-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0530-0