Abstract
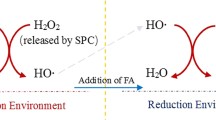
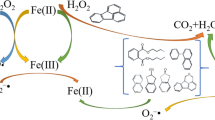
This work demonstrates the impact of hydroxylamine hydrochloride (HAH) addition on enhancing the degradation of trichloroethene (TCE) by the citric acid (CA)-chelated Fe(II)-catalyzed percarbonate (SPC) system. The results of a series of batch-reactor experiments show that TCE removal with HAH addition was increased from approximately 57 to 79% for a CA concentration of 0.1 mM and from 89 to 99.6% for a 0.5 mM concentration. Free-radical probe tests elucidated the existence of hydroxyl radical (HO•) and superoxide anion radical (O2 •-) in both CA/Fe(II)/SPC and HAH/CA/Fe(II)/SPC systems. However, higher removal rates of radical probe compounds were observed in the HAH/CA/Fe(II)/SPC system, indicating that HAH addition enhanced the generation of both free radicals. In addition, increased contribution of O2 •- in the HAH/CA/Fe(II)/SPC system compared to the CA/Fe(II)/SPC system was verified by free-radical scavengers tests. Complete TCE dechlorination was confirmed based on the total mass balance of the released Cl− species. Lower concentrations of formic acid were produced in the later stages of the reaction for the HAH/CA/Fe(II)/SPC system, suggesting that HAH addition favors complete TCE mineralization. Studies of the impact of selected groundwater matrix constituents indicate that TCE removal in the HAH/CA/Fe(II)/SPC system is slightly affected by initial solution pH, with higher removal rates under acidic and near neutral conditions. Although HCO3 − was observed to have an adverse impact on TCE removal for the HAH/CA/Fe(II)/SPC system, the addition of HAH reduced its inhibitory effect compared to the CA/Fe(II)/SPC system. Finally, TCE removal in actual groundwater was much significant with the addition of HAH to the CA/Fe(II)/SPC system. The study results indicate that HAH amendment has potential to enhance effective remediation of TCE-contaminated groundwater.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Buxton G, Greenstock C, Helman W, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Phys Chem Ref Data 17(2):513–886
Chen L, Ma J, Li X, Zhang J, Fang J, Guan Y, Xie P (2011) Strong enhancement on Fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles. Environ Sci Technol 45(9):3925–3930
Choi H, Lim H, Kim J, Hwang T, Kang J (2002) Transport characteristics of gas phase ozone in unsaturated porous media for in-situ chemical oxidation. J Contam Hydrol 57(1):81–98
Elshafei G, Yehia F, Dimitry O, Badawi A, Eshaq G (2010) Degradation of nitrobenzene at near neutral pH using Fe2+-glutamate complex as a homogeneous Fenton catalyst. Appl Catal B Environ 99(1):242–247
Fu X, Gu X, Lu S, Miao Z, Xu M, Zhang X, Qiu Z, Sui Q (2015) Benzene depletion by Fe2+-catalyzed sodium percarbonate in aqueous solution. Chem Eng J 267:25–33
Fu X, Gu X, Lu S, Sharma VK, Brusseau ML, Xue Y, Danish M, Fu GY, Qiu Z, Sui Q (2017) Benzene oxidation by Fe (III)-activated percarbonate: matrix-constituent effects and degradation pathways. Chem Eng J 309:22–29
Gu X, Lu S, Fu X, Qiu Z, Sui Q, Guo X (2017) Carbon dioxide radical anion-based UV/S2O8 2−/HCOOH reductive process for carbon tetrachloride degradation in aqueous solution. Sep Purif Technol 172:211–216
Haag W, Yao C (1992) Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ Sci Technol 26(5):1005–1013
Interstate Technology and Regulatory Council (ITRC) (2002) DNAPL source reduction: facing the challenge. ITRC/DNAPLs-2
Interstate Technology and Regulatory Council (ITRC) (2005) Technical and regulatory guidance for in situ chemical oxidation of contaminated soil and groundwater, Second Edition. ITRC/ISCO-2
Khan N, Adewuyi Y (2010) Absorption and oxidation of nitric oxide (NO) by aqueous solutions of sodium persulfate in a bubble column reactor. Ind Eng Chem Res 49(18):8749–8760
Krembs F, Siegrist R, Crimi M, Furrer R, Petri B (2010) ISCO for groundwater remediation: analysis of field applications and performance. Ground Water Monit Remidiat 30(4):42–53
Liang C, Wang Z, Mohanty N (2006) Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20°C. Sci Total Environ 370(2):271–277
Liang C, Wang Z, Bruell C (2007) Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 66(1):106–113
Liang S, Chen K, Wu C, Lin Y, Kao C (2014) Development of KMnO4-releasing composites for in situ chemical oxidation of TCE-contaminated groundwater. Water Res 54:149–158
Lind J, Merényi G (2006) Kinetic and thermodynamic properties of the aminoxyl (NH2O•) radical. J Phys Chem A 110(1):192–197
Liu H, Bruton T, Doyle F, Sedlak D (2014) In situ chemical oxidation of contaminated groundwater by persulfate: decomposition by Fe(III)-and Mn(IV)-containing oxides and aquifer materials. Environ Sci Technol 48(17):10330–10336
Miao Z, Gu X, Lu S, Dionysiou D, Al-Abed S, Zang X, Wu X, Qiu Z, Sui Q, Danish M (2015a) Mechanism of PCE oxidation by percarbonate in chelated Fe(II)-based catalyzed system. Chem Eng J 275:53–62
Miao Z, Gu X, Lu S, Brusseau ML, Yan N, Qiu Z, Sui Q (2015b) Enhancement effects of reducing agents on the degradation of tetrachloroethene in the Fe(II)/Fe(III) catalyzed percarbonate system. J Hazard Mater 300:530–537
National Research Council (2013) Alternatives for managing the nation’s complex contaminated groundwater sites. National Academies Press, Washington, D.C.
Pignatello J, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36(1):1–84
Rosas J, Vicente F, Saguillo E, Santos A, Romero A (2014) Remediation of soil polluted with herbicides by Fenton-like reaction: kinetic model of diuron degradation. Appl Catal B Environ 144:252–260
Siegrist R, Crimi M, Simpkin T (2011) In situ chemical oxidation for groundwater remediation. Springer, New York
Sindelar H, Brown M, Boyer T (2014) Evaluating UV/H2O2, UV/percarbonate, and UV/perborate for natural organic matter reduction from alternative water sources. Chemosphere 105:112–118
Stroo H, Leeson A, Marqusee J, Johnson P, Ward C, Kavanaugh M, Sale T, Newell C, Pennell K, Lebrón C, Unger M (2012) Chlorinated ethene source remediation: lessons learned. Environ Sci Technol 46:6438–6447
Tamura H, Goto K, Yotsuyanagi T, Nagayama M (1974) Spectrophotometric determination of iron (II) with 1, 10-phenanthroline in the presence of large amounts of iron (III). Talanta 21(4):314–318
Tsitonaki A, Petri B, Crimi M, Mosbæk H, Siegrist R, Bjerg P (2010) In situ chemical oxidation of contaminated soil and groundwater using persulfate: a review. Crit Rev Environ Sci Technol 40(1):55–91
U.S. Environmental Protection Agency (EPA) (2003) The DNAPL remediation challenge: is there a case for source depletion? EPA 600-R-03-143
U.S. Environmental Protection Agency (EPA) (2009) National primary drinking water regulations. EPA 816-F-09-004
Valentine RL, Wang HA (1998) Iron oxide surface catalyzed oxidation of quinoline by hydrogen peroxide. J Environ Eng 124(1):31–38
Viisimaa M, Goi A (2014) Use of hydrogen peroxide and percarbonate to treat chlorinated aromatic hydrocarbon-contaminated soil. J Environ Eng Landsc 22(1):30–39
Zang X, Gu X, Lu S, Miao Z, Zhang X, Fu X, Fu G, Qiu Z, Sui Q (2016) Enhanced degradation of trichloroethene by sodium percarbonate activated with Fe (II) in the presence of citric acid. Water Sci Tech-W Sup. https://doi.org/10.2166/ws.2016.117
Zhang X, Gu X, Lu S, Miao Z, Xu M, Fu X, Qiu Z, Sui Q (2015) Degradation of trichloroethylene in aqueous solution by calcium peroxide activated with ferrous ion. J Hazard Mater 284:253–260
Zou J, Ma J, Chen L, Li X, Guan Y, Xie P, Pan C (2013) Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ Sci Technol 47(20):11685–11691
Funding
This study was financially supported by grants from the National Natural Science Foundation of China (41373094, 21577033, and 51208199), Natural Science Foundation of Shanghai (16ZR1407200). The contributions of Mark Brusseau were supported by the NIEHS Superfund Research Program (P42 ES04940).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Vítor Pais Vilar
Electronic supplementary material
ESM 1
(DOC 320 kb).
Rights and permissions
About this article
Cite this article
Fu, X., Brusseau, M.L., Zang, X. et al. Enhanced effect of HAH on citric acid-chelated Fe(II)-catalyzed percarbonate for trichloroethene degradation. Environ Sci Pollut Res 24, 24318–24326 (2017). https://doi.org/10.1007/s11356-017-0070-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0070-7