Abstract
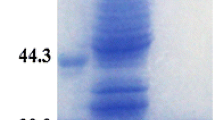
The emissions of CO2 into the atmosphere have been constantly rising due to anthropogenic activities, which have led to global warming and climate change. Among various methods proposed for mitigating CO2 levels in the atmosphere, carbonic anhydrase (CA)-mediated carbon sequestration represents a greener and safer approach to capture and convert it into stable mineral carbonates. Despite the fact that CA is an extremely efficient metalloenzyme that catalyzes the hydration of CO2 (CO2 + H2O ↔ HCO3 − + H+) with a kcat of ∼106 s−1, a thermostable, and alkalistable CA is desirable for the process to take place efficiently. The purified CA from alkaliphilic, moderately thermophilic, and halotolerant Bacillus halodurans TSLV1 (BhCA) is a homodimeric enzyme with a subunit molecular mass of ~37 kDa with stability in a broad pH range between 6.0 and 11.0. It has a moderate thermostability with a T1/2 of 24.0 ± 1.0 min at 60 °C. Based on the sensitivity of CA to specific inhibitors, BhCA is an α-CA; this has been confirmed by nucleotide/amino acid sequence analysis. This has a unique property of stimulation by SO4 2−, and it remains unaffected by SO3 2−, NOx, and most other components present in the flue gas. BhCA is highly efficient in accelerating the mineralization of CO2 as compared to commercial bovine carbonic anhydrase (BCA) and is also efficient in the sequestration of CO2 from the exhaust of petrol driven car, thus, a useful biocatalyst for sequestering CO2 from flue gas.






Similar content being viewed by others
References
Barabesi C, Galizzi A, Mastromei G, Rossi M, Tamburini E, Perito B (2007) Bacillus subtilis gene cluster involved in calcium carbonate biomineralization. J Bacteriol 189:228–235
Bhattacharya S, Schiavone M, Chakrabarti S, Bhattacharya SK (2003) CO2 hydration by immobilized carbonic anhydrase. Biotechnol Appl Biochem 38:111–117
Bond GM, Stringer J, Brandvold DK, Simsek AM, Margaret-Gail EG (2001) Development of integrated system for biomimetic CO2 sequestration using the enzyme carbonic anhydrase. Energy Fuels 15:309–316
Bonra K, Ekrem O (2010) Thermal stability of carbonic anhydrase immobilized within polyurethane foam. Amer Inst Chem Engs 26:1474–1480
Ciullo PA Industrial minerals and their uses (1996) In: Ciullo PA (ed) A handbook and formulatory. William Andrew Publishing/Noyes, New Jersy, pp 17–82
Cowan RM, Ge JJ, Qin YJ, McGregor ML, Trachtenberg MC (2003) CO2 capture by means of an enzyme-based reactor. Ann New York Acad Sci 984:453–469
Cuesta-Seijo JA, Borchert MS, Navarro-Poulsen JC, Schnorr KM, Mortensen SB, Leggio LL (2015) Structure of a dimeric fungal α type carbonic anhydrase. FEBS Lett 585:1042–1048
Dey G, Palit S, Banerjee R, Maiti BR (2002) Purification and characterization of maltooligosaccharide-forming amylase from Bacillus circulans GRS 313. J Indust Microbiol Biotechnol 28:193–200
Ercole C, Cacchio P, Botta AL, Centi V, Lepidi A (2007) Bacterially induced mineralization of calcium carbonate: the role of exopolysaccharide sand capsular polysaccharides. Microsc Microanal 13:42–50
Exhaust gas. Wikipedia, The Free Encyclopedia https://en.wikipedia.org/wiki/Exhaust_gas#cite_note-AUDIEmiss1-11. Assessed February 2, 2016
Faridi S, Satyanarayana T (2015a) Prospects in biomimetic carbon sequestration. In: Goel M, Sudhakar M, Sahi RV (eds) Carbon capture, storage and utilization: a possible climate change solution for energy industry. TERI Press, New Delhi, pp 167–187
Faridi S, Satyanarayana T (2015b) Applicability of carbonic anhydrase in mitigating global wariming and development of useful products from CO2. Clim Change Environm Sustain 3:77–92
Faridi S, Satyanarayana T (2015). Bioconversion of industrial CO2 emissions into utilizable products by employing microbes and their enzymes, In: Chandra R (ed) Environmental waste management, CRC Press, pp 111–156
Ghosh S, Ray M, Ray S (2004) Involvement of histidine residue His382 in pH regulation of MCT4 activity. Indian J Chem Biophys 41:7–13
Hou WC, Chen HJ, Lin YH (2000) Dioscorins from different Dioscorea species all exhibit both carbonic anhydrase and trypsin inhibitor activities. Bot Bull Acad Sinica 41:191–196
Iluata I, Larachi F (2012) New scrubber concept for catalytic CO2 hydration by immobilized carbonic anhydrase II and in-situ inhibitor removal in three-phase monolith slurry reactor. Sep Purif Technol 86:199–214
IPCC Special Report Carbon Dioxide Capture and Storage Summary for Policymakers (2011) Intergovernmental Panel on Climate Change
James P, Isupov MN, Sayer C, Saneei V, Berg S, Lioliou M, Kotlar HK, Littlechild JA (2014) The structure of a tetrameric α-carbonic anhydrase from Thermovibrio ammonificans reveals a core formed around intermolecular disulfides that contribute to its thermostability. Acta Cryst 70:2607–2618
Jo BH, Kim IM, Seo JH, Kang DG, Chaa HJ (2013) Engineered Escherichia coli with periplasmic carbonic anhydrase as a biocatalyst for CO2 sequestration. Appl Environ Microbiol 79:6697–6705
Karabencheva-Christova TG, Carlsson U, Balali-Mood K, Black GW, Christov CZ (2013) Unoconformational effects on the Circular Dichroism of human carbonic anhydrase II: a multilevel computational study. PLoS One 8:e56874
Kaur S, Bhattacharya A, Sharma A, Tripathi AK (2012) Diversity of microbial carbonic anhydrases, their physiological role and applications. In: Johri BN, Prakash A (eds) Satyanarayana T. Springer, Microorganisms in environmental management, pp 151–173
Khalifah RG, Silverman DN (1991) In: Dodgson SJ, Tashian RE, Gros G, Carter ND (eds) The carbonic anhydrases: cellular physiology and molecular genetics. Plenum Press, New York, pp 49–70
Kumar V, Satyanarayana T (2013) Biochemical and thermodynamic characteristics of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 17:797–808
Lane TW, Saito MA, George GN, Pickering IJ, Prince RC, Orel FMM (2000) Biochemistry: a cadmium enzyme from a marine diatom. Nature 435:42
Laois-Jeune C, Andrade-Navarro MA, Perez-Iratxeta C (2011) Prediction of proteins secondary structure from circular dichroism using theoretically derived spectra., Wiley periodicals, http://www.ogic.ca/projects/k2d3/
Lee SW, Park SB, Jeong SK, Lim KS, Lee SH, Trachtenberg MC (2010) On carbon dioxide storage based on biomineralization strategies. Micron 41:273–282
Li L, Fu M, Zhaou Y, Zhu Y (2012) Characterization of carbonic anhydrase II from Chlorella vulgaris in bio-CO2 capture. Environ Sci Pollut Res 19:4227–4232
Liu W, Shi P, Chen Q, Yang P, Wang G, Wang Y, Luo H, Yao B (2010) Gene cloning, over-expression, and characterization of a xylanase from Penicillium sp. CGMCC 1699. Appl Biochem Biotechnol 162:1–12
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Meeting C, Nguyen. Low-Cost Enzyme-Based Technology for Carbon Capture. (https://www.netl.doe.gov/File%20Library/Events/2012/CO2%20Capture%20Meeting/L-Nguyen-Codexis-ARPA-e-Enzyme-based-Capture.pdf)
Mikulski RL, Silverman DN (2010) Proton transfer in catalysis and the role of proton shuttles in carbonic anhydrase. Biochim Biophys Acta 1804:422–426
Mirjafari P, Asghari K, Mahinpey N (2007) Investigating the application of enzyme carbonic anhydrase for CO2 sequestration purposes. Ind Eng Chem Res 46:921–926
Nisha M, Satyanarayana T (2013) Characterization of recombinant amylopullulanase (gt-apu) and truncated amylopullulanase (gt-apuT) of the extreme thermophile Geobacillus thermoleovorans NP33 and their action in starch saccharification. Appl Microbiol Biotechnol 97:6279–6292
Oviya M, Giri SS, Sukumaran V, Natarajan P (2012) Immobilization of carbonic anhydrase enzyme purified from Bacillus subtilis VSG-4 and its application as CO2 sequesterer. Prep Biochem Biotech 42:462–475
Oviya M, Sukumaran V, Giri SS (2013) Immobilization and characterization of carbonic anhydrase purified from E. coli MO1 and its influence on CO2 sequestration. World J Microbiol Biotechnol 29:1813–1820
Park H, Song B, Morel FM (2007) Diversity of the cadmium-containing carbonic anhydrase in marine diatoms and natural waters. Environ Microbiol 9:403–413
Pomar L, Hallock (2008) Carbonate factories: a conundrum in sedimentary geology. Earth Sci Rev 287:134–169
Prabhu C, Wanjari S, Gawande S, Das S, Labhsetwar N, Kotwal S, Puri AK, Satyanarayana T, Rayalu S (2009) Immobilization of carbonic anhydrase enriched microorganism on biopolymer based materials. J Mol Catal B Enzym 60:13–21
Prabhu C, Wanjari S, Puri A, Bhattacharya A, Pujari R, Yadav R, Das S, Labhsetwar N, Sharma A, Satyanarayana Z, Rayalu S (2011) Region specific bacterial carbonic anhydrase for biomimetic sequestration of carbon dioxide. Energy Fuels 25:1327–1332
Pushkas LG, Inui M, Zahan K, Yukawa H (2000) A periplasmic, α-type carbonic anhydrase from Rhodopseudomonas palustris is essential for bicarbonate uptake. Microbiology 146:2957–2966
Ramanan R, Kannan K, Sivanesan SD (2009) Biosequestration of carbon dioxide using carbonic anhydrase enzyme purified from Citrobacter freundii. World J Microbiol Biotechnol 25:981–987
Sharma A, Bhattacharya A (2010) Enhanced biomimetic sequestration of CO2 into CaCO3 using purified carbonic anhydrase from indigenous bacterial strains. J Mol Catal B Enzym 67:122–128
Sharma A, Bhattacharya A, Singh S (2009) Purification and characterization of an extracellular carbonic anhydrase from Pseudomonas fragi. Process Biochem 44:1293–1297
Sharma A, Satyanarayana T (2012) Cloning and expression of acidstable, high maltose-forming, Ca2+ independent α-amylase from an acidophile Bacillus acidicola and its applicability in starch hydrolysis. Extremophiles 16:515–522
Smith KS, Ferry JG (2000) Prokaryotic carbonic anhydrases. FEMS Microbiol Rev 24:335–366
Steiner H, Jonsson BH, Lindskog S (1975) The catalytic mechanism of carbonic anhydrase. Hydrogen-isotope effects on the kinetic parameters of the human C isoenzyme. Eur J Biochem 59:253–259
Tashian RE (1989) The carbonic anhydrases: widening perspectives on their evolution, expression and function. BioEssays 10:186–192
Tripp BC, Smith K, Ferry JG (2001) Carbonic anhydrase: new insights for an ancient enzyme. J Biol Chem 276:48615–48618
Verpoorte JA, Mehta SJ, Edsall T (1967) Esterase activities of human carbonic anhydrases. B and C J Biol Chem 242:4221–4229
Vinoba M, Kim DH, Lim KS, Jeong SK, Lee SW, Alagar M (2011) Biomimetic sequestration of CO2 and reformation to CaCO3 using bovine carbonic anhydrase immobilized on SBA-15. Energy Fuels 25:438–445
Wilkins RG, Williams KR (1974) Kinetics of formation and dissociation of manganese-bovine carbonic anhydrase. B J Am Chem Soc 96:2241–2245
Yadav R, Wanjari S, Prabhu C, Vivek K, Labhsetwar N, Satyanarayana T, Kotwal S, Rayalu S (2010) Immobilized carbonic anhydrase for the biomimetic carbonation reaction. Energy Fuels 24:6198–6207
Yanyou W, Xinzhen Z, Pingping L, Huakun H (2007) Impact of Zn, Cu and Fe on the activity of carbonic anhydrase of erythrocytes in ducks. Biol Trace Elem Res 118:227–232
Acknowledgments
One of us (SF) is grateful to the University of Delhi, Delhi, for the award of fellowship during the course of this investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Santiago V. Luis
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 959 kb)
Rights and permissions
About this article
Cite this article
Faridi, S., Satyanarayana, T. Novel alkalistable α-carbonic anhydrase from the polyextremophilic bacterium Bacillus halodurans: characteristics and applicability in flue gas CO2 sequestration. Environ Sci Pollut Res 23, 15236–15249 (2016). https://doi.org/10.1007/s11356-016-6642-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6642-0