Abstract
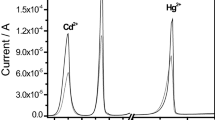
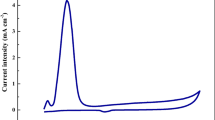
A simple cyclic voltammetry (CV) analytical method with organo-modified sericite for the working electrode was investigated to detect As(III) in an aquatic environment, and optimal conditions for the reliable measurement of trace amounts of As(III) were studied. A distinct, specific peak was clearly observed at 0.8 V due to the reduction of H3AsO4 to H3AsO3. The specific peak current of arsenic increased with increasing the concentration of As(III) and initially increased proportionally to the scan rates. However, it disappeared as the scan rate increased over 400 mV/s. Because the surface of the organo-modified sericite electrode rapidly became saturated with As(III) when the deposition time increased, an optimal deposition time was determined as 60 s. Pb2+ had no significant influence on the peak signal of As(III), whereas it was reduced as the ratio of Cu/As increased. Considering the detection limit of arsenic (1 ppb), this system can be used to detect low levels of As(III) in water systems.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ahmad SA, Bandaranayake D, Khan AW, Hadi SA, Uddin G, Halim MA (1997) Arsenic contamination in ground water and asenicosis in Bangladesh. Int J Environ Health Res 7:271–276
Buchet JP, Lauwerys R (1994) Interpretation of inorganic arsenic metabolism in humans in the light of observations made in vitro and in vivo in the rat. Appl Organomet Chem 8:191–196
Dirilgen N, Dogan F, Ozbal H (2006) Anodic stripping voltammetry: arsenic determination in ancient bone samples. Anal Lett 39:127–143
Gupta P, Goyal RN (2014) Polymelamine modified edge plane pyrolytic graphite sensor for the electrochemical assay of serotonin. Talanta 120:17–22
Konta J (1995) Clay and man: clay raw materials in the service of man. Appl Clay Sci 10:275–355
Kopanica M, Novotný L (1998) Determination of traces of arsenic(III) by anodic stripping voltammetry in solutions, natural waters and biological material. Anal Chim Acta 368:211–218
Lee SM, Lalhmunsiama TD (2014) Sericite in the remediation of Cd(II) and Mn(II) contaminated waters: Batch and column studies. Environ Sci Pollut Res 21:3686–3696
Lee SM, Tiwari D (2012) Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: an overview. Appl Clay Sci 59–60:84–102
Lee SM, Tiwari D (2014) Organo-modified sericite in the remediation of aquatic environment contaminated with As(III) or As(V). Environ Sci Pollut Res 21:407–418
Lee HS, Seo JH, Lee KR, Yun TK (2003) Continuous biosorption of heavy metal ions by a seaweed in fixed bed column. Environ Eng Res 25:832–837
Manisankar P, Selvanathan TG, Vedhi C (2005) Utilization of sodium montmorillonite clay-modified electrode for the determination of isoproturon and carbendazim in soil and water samples. Appl Clay Sci 29:249–257
Markarian J (2005) Automotive and packaging offer growth opportunities for nanocomposites. Plast Addit Compound 7(6):18–25
Peacock CL, Shermon DM (2005) Surface complexation model for multisite adsorption of copper(II) onto kaoloniote. Geochim Cosmochim Acta 69:3733–745
Svancara I, Vytras K, Bobrowsky A, Kalcher K (2002) Determination of arsenic at a gold-plated carbon paste electrode using constant current stripping analysis. Talanta 58:45–55
Tiwari D, Prasad SK, Yang JK, Choi BJ, Lee SM (2006) Inorganic and bio-materials in the removal/speciation of radiocesium and radiostrontium: an overview. Environ Eng Res 11:106–125
Tiwari D, Laldawngliana C, Lee SM (2014a) Immobilized small sized manganese dioxide sand in the remediation of arsenic contaminated water. Environ Eng Res 19:107–113
Tiwari D, Lalhmunsiama CSI, Lee SM (2014b) Activated sericite: an efficient and effective natural material in attenuation of cesium from aquatic environment. Pedosphere 24:731–742
Tonle IK, Ngameni E, Tcheumi HL, Tchieda V, Carteret C, Walcarius C (2008) Sorption of methylene blue on an organoclay bearing thiol groups and application to electrochemical sensing of the dye. Talanta 74:489–497
U. S. Environmental Protection Agency (2007) www.epa.gov/safewater/arsenic.html
Welch AH, Westjohn DB, Helsel DR, Wanty RB (2000) Arsenic in ground water of the United States: occurrence and geochemistry. Ground Water 38:589–604
World Health Organization (2004) www.WHO.int/water sanitation health/water quality/arsenic.html
Xi Y, Ding Z, Honping H (2005) Infrared spectroscopy of organo clays synthesized with the surfactant octadecyltrimethylammonium bromide. Spectrochim Acta Part A 61:515–525
Yang Z, Ren AM, Zou LY, Guo JF, Feng JK (2011) A theoretical study on magnesium ion–selective two-photon fluorescent probe based on benzo [h] chromene derivatives. Theor Chem Accounts 130:61–68
Acknowledgments
This work was supported by the Korea Ministry of Environment as “Converging technology project” (Proposal No. 2013001450001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kim, MN., Yang, JK., Park, YJ. et al. Application of a novel electrochemical sensor containing organo-modified sericite for the detection of low-level arsenic. Environ Sci Pollut Res 23, 1044–1049 (2016). https://doi.org/10.1007/s11356-015-5747-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5747-1