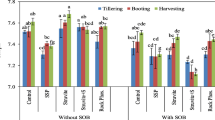
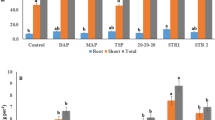
Abstract
Struvite precipitate obtained from yeast industry anaerobic effluent with high ammonium nitrogen (NH4–N) was investigated for fertilizer effect on plant growth and nutrition according to applications of N, nitrogen/phosphorus/potassium (NPK), and control. Optimum struvite formation conditions were determined via Box–Behnken design. Optimum condition was obtained at pH 9.0 and Mg/N/P molar ratio of 1.5:1:1. Under these conditions, heavy metal concentrations in the obtained struvite precipitate (except Cu) were below the detection limits. In addition to high N, P, and Mg content, energy-dispersive X-ray (EDX) analysis showed that the struvite also included the nutritional elements Ca, K, Na, and Fe. X-ray diffraction (XRD) analysis revealed the complex structures of NaAl(SO4)2(H2O)12, NaMn2+Fe2(PO4)3, and (Na2,Ca)O2(Fe,Mn)O.P2O5 in the precipitate. High Na+ and Ca2+ concentrations in the anaerobic effluent reacted with phosphate during struvite precipitation. Different applications and struvite dosages significantly affected fresh and dry weights and nutrient element uptakes by plants (P < 0.05). N, P, and Mg uptakes of plants were significantly higher at struvite ×2, ×3, and ×4 dosages compared with NPK application. For adequate nutrition and supply of optimum dry weight, struvite ×2 dosage (5.71 g struvite/kg soil) was found appropriate for both maize and tomato plants.




Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- COD:
-
Chemical oxygen demand
- EDX:
-
Energy-dispersive X-ray analyzer
- HSD:
-
Honestly significant difference
- R 2 :
-
Determination coefficient
- SCOD:
-
Soluble chemical oxygen demand
- TCOD:
-
Total chemical oxygen demand
- TS:
-
Total solid
- TSS:
-
Total suspended solid
- x i :
-
Coded value of the ith factor
- X i :
-
Real value of the ith factor
- X 0 :
-
Real value of the center point
- XRD:
-
X-ray diffraction
- Y :
-
Removed NH4–N or PO4–P percentage
References
APHA AWWA WEF (2005) Standard methods for the examination of water and wastewater, 21st ed., Washington, DC
Black CA (2002) Methods of soil analysis, part 2. American Society of Agronomy, Madison
Çatalkaya EÇ, Şengül F (2006) Application of Box–Wilson experimental design method for the photodegradation of bakery’s yeast industry with UV/H2O2 and UV/H2O2/Fe(II) process. J Hazard Mater B128:201–207
Cojocaru C, Zakrzewska-Trznadel G, Miskiewicz A (2009) Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. Part 1: optimization of complexation conditions. J Hazard Mater 169:610–620
de-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38:4222–4246
Deveci N, Çiftçi G (2001) A mathematical model for the anaerobic treatment of Baker’s yeast effluents. Waste Manag 21:99–103
Diwani GE, Rafie SE, Ibiari NNE, El-Aila HI (2007) Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer. Desalination 214:200–214
Doyle JD, Parsons SA (2002) Struvite formation, control and recovery. Water Res 36:3925–3940
Ferraris G, Fuess H, Joswig W (1986) Neutron diffraction study of MgNH4PO4·6H2O (struvite) and survey of water molecules donating short hydrogen bonds. Acta Crystallogr B42:253–258
Gaterell MR, Gay R, Wilson R, Gochin RJ, Lester JN (2000) An economic and environmental evaluation of the opportunities for substituting phosphorus recovered from wastewater treatment works in existing UK fertiliser markets. Environ Technol 21:1067–1084
Huang H, Xu C, Zhang W (2011) Removal of nutrients piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour Technol 102:2523–2528
Kobya M, Delipinar S (2008) Treatment of the baker’s yeast wastewater by electrocoagulation. J Hazard Mater 154:1133–1140
Kumar R, Pal P (2013) Turning hazardous waste into value-added products: production and characterization of struvite from ammoniacal waste with new approaches. J Clean Prod 43:59–70
Li XZ, Zhao QL (2003) Recovery of ammonium-nitrogen from landfill leachate as a multi-nutrient fertilizer. Ecol Eng 20:171–181
Liu JCW (2009) Recovery of phosphate and ammonium as struvite from semiconductor wastewater. Sep Purif Technol 64:368–373
Montgomery DC (1997) Design and analysis of experiments, 4th edn. Wiley, New York
Pala A, Erden G (2005) Decolorization of a baker’s yeast industry effluent by Fenton oxidation. J Hazard Mater B127:141–148
Pastor L, Marti N, Bouzas A, Seco A (2008) Sewage sludge management for phosphorus recovery as struvite in EBPR wastewater treatment plants. Bioresour Technol 99:4817–4824
Rahman MM, Liu YH, Kwag JH, Ra CS (2011) Recovery of struvite from animal wastewater and its nutrient leaching in soil. J Hazard Mater 186:2026–2030
Ryu HD, Lim CS, Kang MK, Lee SI (2012) Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J Hazard Mater 221–222:248–255
Shu L, Schneider P, Jegatheesan V, Johnson J (2006) An economic evaluation of phosphorus recovery as struvite from digester supernatant. Bioresour Technol 97:2211–2216
Türker M, Çelen I (2007) Removal of ammonia as struvite from anaerobic digester effluents and recycling of magnesium and phosphate. Bioresour Technol 98:1529–1534
Uysal A, Yılmazel YD, Demirer GN (2010) The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J Hazard Mater 181:248–254
Yetilmezsoy K, Sertyesilisik B, Kocak E, Sapci-Zengin Z (2009) Ameliorative effect of different doses of MgNH4PO4·6H2O precipitate recovered from the effluent of UASB treating poultry manure wastewater: growth of Lolium perenne. J Food Agric Environ 7:823–831
Yu R, Geng J, Ren H, Wang Y, Xu K (2012) Combination of struvite pyrolysate recycling with mixed-base technology for removing ammonium from fertilizer wastewater. Bioresour Technol 124:292–298
Zhang T, Ding L, Ren H, Xiong X (2009) Ammonium nitrogen removal of from coking wastewater by chemical precipitation recycle technology. Water Res 43:5209–5215
Zhou Y, Liang Z, Wang Y (2008) Decolorization and COD removal of secondary yeast wastewater effluents by coagulation using aluminum sulfate. Desalination 225:301–311
Acknowledgments
This study was supported by research grants from the Scientific and Technological Council of Turkey (TUBITAK) (project no. 110Y077) and Research Project Funding Unit of the Suleyman Demirel University (project no. 3283-YL1-12).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Uysal, A., Demir, S., Sayilgan, E. et al. Optimization of struvite fertilizer formation from baker’s yeast wastewater: growth and nutrition of maize and tomato plants. Environ Sci Pollut Res 21, 3264–3274 (2014). https://doi.org/10.1007/s11356-013-2285-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2285-6