Abstract
Aim
This study was aimed to consider the effects of uphill (concentric, CON) and downhill (eccentric, ECC) treadmill exercise with Nano-BCAA supplementation on muscle protein expression of Akt and mTOR.
Methods
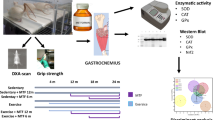
Thirty aging male Wistar rats were randomly divided into 6 groups of (n = 5 each group): control (healthy), uphill running (CON, 0 to + 15°), downhill running (ECC, 0 to − 15°), Nano-BCAA (BCAA with Nano-Chitosan), CON + Nano-BCAA, and ECC + Nano-BCAA. The exercise training was performed in an interval form, with 3 sessions per weeks lasting 8 weeks. BCAA (in Nano form) administered by gavage 3 sessions per week for 8 weeks. RT-PCR was used to measure gene expression of Akt and mTOR. As well, protein expression of mTOR was performed by the IHC method.
Results
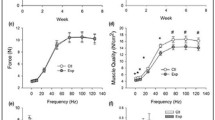
Administration of BCAA with CON and ECC increased the Akt gene expression (p < 0.05). Co-treatment of Nano-BCAA and exercises leads to much higher values of Akt than does single treatment. Compared to the healthy control group (without Nano-BCAA), co-treatment of CON + Nano-BCAA and ECC + Nano-BCAA showed a significant increase in the mTOR gene expression (p < 0.05).
Conclusion
The use of walking exercises, especially with a negative or positive slope, along with proper nutrition (taking healthy supplements such as BCAA) could be effective in strengthening muscle tissue, especially at the cellular level (increasing the Akt/mTOR activity). It can be an optimal alternative for those who cannot use resistance training at old age.




Similar content being viewed by others
References
Robinson L (2018) Successful ageing. Lancet 391(10118):300
Sander M et al (2015) The challenges of human population ageing. Age Ageing 44(2):185–187
Luo J et al (2020) Ageing, age-related diseases and oxidative stress: what to do next? Ageing Res Rev 57:100982
Larsson L et al (2019) Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99(1):427–511
Santilli V et al (2014) Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 11(3):177
Janssen I (2010) Evolution of sarcopenia research. Appl Physiol Nutr Metab 35(5):707–712
Tournadre A et al (2019) Sarcopenia. Joint Bone Spine 86(3):309–314
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52:80–85
Kumar V et al (2009) Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 106(6):2026–2039
Phillips SM, Hartman JW, Wilkinson SB (2005) Dietary protein to support anabolism with resistance exercise in young men. J Am Coll Nutr 24(2):134S-139S
Little JP, Phillips SM (2009) Resistance exercise and nutrition to counteract muscle wasting. Appl Physiol Nutr Metab 34(5):817–828
Glover EI, Phillips SM (2010) Resistance exercise and appropriate nutrition to counteract muscle wasting and promote muscle hypertrophy. Curr Opin Clin Nutr Metab Care 13(6):630–634
Dardevet D et al (2012) Muscle wasting and resistance of muscle anabolism: the “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci World J 2(26):1–6
Bodine SC et al (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3(11):1014–1019
Jefferson LS, Fabian JR, Kimball SR (1999) Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int J Biochem Cell Biol 31(1):191–200
Shah OJ et al (2000) 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279(4):E715–E729
Cohen S, Nathan JA, Goldberg AL (2015) Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 14(1):58–74
Manning BD (2010) Insulin signaling: inositol phosphates get into the Akt. Cell 143(6):861–863
Lai K, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ (2004) Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24:9295–9304
Bentzinger CF et al (2008) Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8(5):411–424
Léger B et al (2006) Akt signalling through GSK-3β, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576(3):923–933
Pratiwi YS et al (2018) Nutmeg extract increases skeletal muscle mass in aging rats partly via IGF1-AKT-mTOR pathway and inhibition of autophagy. Evidence-Based Complement. Altern Med 2(8):1–8
Fry CS et al (2011) Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1(1):1–11
Francaux M et al (2016) Aging reduces the activation of the mTORC1 pathway after resistance exercise and protein intake in human skeletal muscle: potential role of REDD1 and impaired anabolic sensitivity. Nutrients 8(1):47
Breen L, Phillips SM (2011) Skeletal muscle protein metabolism in the elderly: interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab 8(1):68
Morino K et al (2005) IRS-1 serine phosphorylation is a key molecular event in the pathogenesis of fat-induced insulin resistance in skeletal muscle in vivo. Diabetes 54:A339
Pansarasa O et al (2008) Oral amino acid supplementation counteracts age-induced sarcopenia in elderly rats. Am J Cardiol 101(11):S35–S41
Eley HL, Russell ST, Tisdale MJ (2007) Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem J 407(1):113–120
Hanai T et al (2015) Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 31(1):193–199
Ko C-H et al (2020) Effects of enriched branched-chain amino acid supplementation on sarcopenia. Aging 12(14):15091
Abdel-Tawwab M, Razek NA, Abdel-Rahman AM (2019) Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol 88:254–258
Kim J-S, Yi H-K (2015) Intermittent bout exercise training down-regulates age-associated inflammation in skeletal muscles. Exp Gerontol 72:261–268
Luo L et al (2013) Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol 48(4):427–436
Lewis EJ et al (2013) Mild eccentric exercise increases Hsp72 content in skeletal muscles from adult and late middle-aged rats. Cell Stress Chaperones 18(5):667–673
Kaux JF et al (2013) Eccentric training improves tendon biomechanical properties: a rat model. J Orthop Res 31(1):119–124
Hortobágyi T et al (2011) Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol Ser A Biomed Sci Med Sci 66(5):541–547
Gojda J et al (2017) Increased incretin but not insulin response after oral versus intravenous branched chain amino acids. Ann Nutr Metab 70(4):293–302
Mirdar S et al (2019) The effects of tapering with and without ethanolic extract of Nigella sativa on Hypoxia Inducible Factor-1α and exercise-induced bronchial changes. J Mil Med 21(2):131–141
Callahan DM, Kent-Braun JA (2011) Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol 111(5):1345–1352
Shirvani H et al (2021) Swimming exercise improves gene expression of PPAR-γ and downregulates the overexpression of TLR4, MyD88, IL-6, and TNF-α after high-fat diet in rat skeletal muscle cells. Gene 775:145441
McPhee JS et al (2016) Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology 17(3):567–580
Zhao J et al (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6(6):472–483
Zou K et al (2011) The α7β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol 111(4):1134–1141
Malm C et al (2004) Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol 556(3):983–1000
Franz JR, Kram R (2013) How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture 37(3):378–384
Gallagher P et al (2007) Interaction of resistance exercise and BCAA supplementation on Akt and p70 s6 kinase phosphorylation in human skeletal muscle. Exp Biol 21(6):1206–1212
Kimball SR, Jefferson LS (2006) Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 136(1):227S-231S
Tayebi SM et al (2019) Plasma retinol-binding protein-4 and tumor necrosis factor-α are reduced in postmenopausal women after combination of different intensities of circuit resistance training and Zataria supplementation. Sport Sci Health 15(3):551–558
Bolster DR, Jefferson LS, Kimball SR (2004) Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc 63(2):351–356
Wilkinson DJ et al (2013) Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 591(11):2911–2923
Guridi M et al (2016) Alterations to mTORC1 signaling in the skeletal muscle differentially affect whole-body metabolism. Skelet Muscle 6(1):1–14
Aagaard P et al (2000) Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol 89(6):2249–2257
Westing SH et al (1988) Eccentric and concentric torque-velocity characteristics of the quadriceps femoris in man. Eur J Appl Physiol 58(1–2):100–104
Armstrong R, Ogilvie R, Schwane J (1983) Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol 54(1):80–93
Gordon A, Huxley AF, Julian F (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184(1):170–192
Lynn R, Morgan D (1994) Decline running produces more sarcomeres in rat vastus intermedius muscle fibers than does incline running. J Appl Physiol 77(3):1439–1444
Franz JR, Kram R (2014) Advanced age and the mechanics of uphill walking: a joint-level, inverse dynamic analysis. Gait Posture 39(1):135–140
da Rocha AL et al (2015) Downhill running-based overtraining protocol improves hepatic insulin signaling pathway without concomitant decrease of inflammatory proteins. PLoS ONE 10(10):e0140020
Da Rocha AL et al (2016) Downhill running excessive training inhibits hypertrophy in mice skeletal muscles with different fiber type composition. J Cell Physiol 231(5):1045–1056
Takegaki J et al (2020) The effect of leucine-enriched essential amino acid supplementation on anabolic and catabolic signaling in human skeletal muscle after acute resistance exercise: a randomized, double-blind, placebo-controlled, parallel-group comparison trial. Nutrients 12(8):2421
Borgenvik M, Apró W, Blomstrand E (2012) Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am J Physiol Endocrinol Metab 302(5):E510–E521
Goodman CA, Hornberger TA (2013) Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev 41(2):107
Acknowledgements
No acknowledgements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study protocol was approved by the Institutional Animal Ethics Committee of the University.
Informed consent
Animal study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadri, S., Sharifi, G. & Jalali Dehkordi, K. Nano branched-chain amino acids enhance the effect of uphill (concentric) and downhill (eccentric) treadmill exercise on muscle gene expression of Akt and mTOR on aged rats. Sport Sci Health 18, 481–490 (2022). https://doi.org/10.1007/s11332-021-00828-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-021-00828-6