Abstract
Purpose
We aim to evaluate reactive oxygen species modulator 1 (Romo1) levels in obstructive sleep apnea syndrome (OSAS) and analyze its possible relationships to OSAS severity, reactive oxygen species (ROS), and C-reactive protein (CRP). Additionally, we also investigated the effects of nasal continuous positive airway pressure (nCPAP) on serum Romo1.
Methods
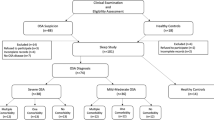
One hundred and five patients diagnosed with OSAS were classified into the OSAS group, and 41 subjects without OSAS were recruited for the control group. The OSAS group was further divided into mild, moderate, and severe OSAS subgroups. Fifteen patients with moderate and severe OSAS were treated with nCPAP. Serum levels of Romo1, ROS, and CRP were also measured.
Results
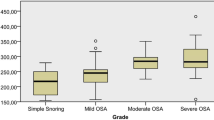
Serum Romo1, ROS, and CRP were the lowest in normal subjects and increased across OSAS severities (P < 0.05). Univariate analysis showed that serum Romo1 was positively correlated with apnea-hypopnea index (AHI), oxygen desaturation index (ODI), time spent below 90% oxygen saturation (Ts90%), arousal index, ROS, and CRP, and was negatively correlated with minimal oxygen saturation (miniSaO2) (all P < 0.05). Multiple linear regression analysis showed that serum Romo1 level was significantly associated with AHI and ODI, after adjusting for age, gender, BMI, and CRP. After 6 months of nCPAP therapy, serum Romo1, ROS, and CRP were significantly decreased (P < 0.05).
Conclusions
The increase of serum Romo1 in OSAS patients was positively correlated with disease severity. Serum Romo1 may be an important parameter for monitoring the severity of OSAS and treatment efficiency.

Similar content being viewed by others
Abbreviations
- OSAS:
-
Obstructive sleep apnea syndrome
- nCPAP:
-
Nasal continual positive airway pressure
- PSG:
-
Polysomnography
- AHI:
-
Apnea-hypopnea index
- ODI:
-
Oxygen desaturation index
- Ts90%:
-
Time spent below 90% oxygen saturation
- BMI:
-
Body mass index
- NC:
-
Neck circumference
- Romo1:
-
Reactive oxygen species modulator 1
- ROS:
-
Reactive oxygen species
- CRP:
-
C-reactive protein
References
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165(9):1217–1239. https://doi.org/10.1164/rccm.2109080
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342(19):1378–1384. https://doi.org/10.1056/NEJM200005113421901
Pedrosa RP, Barros IML, Drager LF, Bittencourt MS, Medeiros AKL, Carvalho LL, Lustosa TC, Carvalho MMB, Ferreira MNL, Lorenzi-Filho G, Costa LOBF (2014) OSA is common and independently associated with hypertension and increased arterial stiffness in consecutive perimenopausal women. Chest 146(1):66–72. https://doi.org/10.1378/chest.14-0097
Levy P, Malcolm K, Walter TM et al (2015) Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 1:15015
McNicholas WT, Bonsigore MR (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29(1):156–178. https://doi.org/10.1183/09031936.00027406
Tan S, Liu X, Xu Y, Luo L, Zhou S, Gao Y (2017) Serum high-density lipoprotein correlates with serum apolipoprotein M and A5 in obstructive sleep apnea hypopnea syndrome. Sleep Breath 21(1):37–44. https://doi.org/10.1007/s11325-016-1357-5
Kent BD, Grote L, Ryan S, Pépin JL, Bonsignore MR, Tkacova R, Saaresranta T, Verbraecken J, Lévy P, Hedner J, McNicholas WT (2014) Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest 146(4):982–990. https://doi.org/10.1378/chest.13-2403
Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS (2014) Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 190(2):218–225. https://doi.org/10.1164/rccm.201312-2209OC
Lipford MC, Flemming KD, Calvin AD, Mandrekar J, Brown RD Jr, Somers VK, Caples SM (2015) Associations between cardioembolic stroke and obstructive sleep apnea. Sleep 38(11):1699–1705. https://doi.org/10.5665/sleep.5146
Mello-Fujita L, Kim LJ, Palombini Lde O et al (2015) Treatment of obstructive sleep apnea syndrome associated with stroke. Sleep Med 16(6):691–696. https://doi.org/10.1016/j.sleep.2014.12.017
DeMartino T, Ghoul RE, Wang L, Bena J, Hazen SL, Tracy R, Patel SR, Auckley D, Mehra R (2016) Oxidative stress and inflammation differentially elevated in objective versus habitual subjective reduced sleep duration in obstructive sleep apnea. Sleep 39(7):1361–1369. https://doi.org/10.5665/sleep.5964
Oyama J, Yamamoto H, Maeda T et al (2012) Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol 35(4):231–236. https://doi.org/10.1002/clc.21010
Hall AB, Ziadi MC, Leech JA, Chen SY, Burwash IG, Renaud J, deKemp RA, Haddad H, Mielniczuk LM, Yoshinaga K, Guo A, Chen L, Walter O, Garrard L, DaSilva JN, Floras JS, Beanlands RSB (2014) Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation 130(11):892–901. https://doi.org/10.1161/CIRCULATIONAHA.113.005893
Sunnetcioglu A, Alp HH, Sertogullarindan B, Balaharoglu R, Gunbatar H (2016) Evaluation of oxidative damage and antioxidant mechanisms in COPD, lung cancer, and obstructive sleep apnea syndrome. Resp Care 61(2):205–211. https://doi.org/10.4187/respcare.04209
Jordan W, Cohrs S, Degner D, Meier A, Rodenbeck A, Mayer G, Pilz J, Rüther E, Kornhuber J, Bleich S (2006) Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transm 113(2):239–254. https://doi.org/10.1007/s00702-005-0316-2
Chen CY, Chen CL, CC Y et al (2015) Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Med 16(1):113–118. https://doi.org/10.1016/j.sleep.2014.07.027
Yildirim T, Alp R (2017) The role of oxidative stress in the relation between fibromyalgia and obstructive sleep apnea syndrome. Eur Rev Med Pharmacol Sci 21(1):20–29
Sosa V, Moline T, Somoza R et al (2013) Oxidative stress and cancer: an overview. Mech Ageing Dev 12(1):376–390
Mancuso M, Bonanni E, LoGerfo A, Orsucci D, Maestri M, Chico L, DiCoscio E, Fabbrini M, Siciliano G, Murri L (2012) Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med 13(6):632–636. https://doi.org/10.1016/j.sleep.2011.10.030
Chung YM, Kim JS, Yoo YD (2006) A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Commun 347(3):649–655. https://doi.org/10.1016/j.bbrc.2006.06.140
Kim JJ, Lee SB, Park JK, Yoo YD (2010) TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L). Cell Death Differ 17(9):1420–1434. https://doi.org/10.1038/cdd.2010.19
Lee SH, Lee JS, Lee EJ, Min KH, Hur GY, Lee SH, Lee SY, Kim JH, Lee SY, Shin C, Shim JJ, Kang KH, In KH (2014) Serum reactive oxygen species modulator 1 (Romo1) as a potential diagnostic biomarker for non-small cell lung cancer. Lung Cancer 85(2):175–181. https://doi.org/10.1016/j.lungcan.2014.05.023
Shin JA, Chung JS, Cho SH, Kim HJ, Yoo YD (2013) Romo1 expression contributes to oxidative stress-induced death of lung epithelial cells. Biochem Biophys Res Commun 439(2):315–320. https://doi.org/10.1016/j.bbrc.2013.07.012
Chung JS, Park S, Park SH et al (2012) Overexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology 143(4):1084–1094 e1087. https://doi.org/10.1053/j.gastro.2012.06.038
Kowluru RA, Kowluru V, Xiong Y, Ho YS (2006) Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radical Biol Med 41(8):1191–1196. https://doi.org/10.1016/j.freeradbiomed.2006.01.012
Chung YM, Lee SB, Kim HJ, Park SH, Kim JJ, Chung JS, Yoo YD (2008) Replicative senescence induced by Romo1-derived reactive oxygen species. J Bio Chem 283(48):33763–33771. https://doi.org/10.1074/jbc.M805334200
Lee SB, Kim JJ, Kim TW, Kim BS, Lee MS, Yoo YD (2010) Serum deprivation-induced reactive oxygen species production is mediated by Romo1. Apoptosis 15(2):204–218. https://doi.org/10.1007/s10495-009-0411-1
Chung JS, Lee SB, Park SH, Kang ST, Na AR, Chang TS, Kim HJ, Yoo YD (2009) Mitochondrial reactive oxygen species originating from Romo1 exert an important role in normal cell cycle progression by regulating p27(Kip1) expression. Free Radic Res 43(8):729–737. https://doi.org/10.1080/10715760903038432
Lavie L (2015) Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev 20:27–45. https://doi.org/10.1016/j.smrv.2014.07.003
Yang CH, Shen YJ, Lai CJ, Kou YR (2016) Inflammatory role of ROS-sensitive AMP-activated protein kinase in the hypersensitivity of lung vagal C fibers induced by intermittent hypoxia in rats. Front Physiol 7:263
Karamanli H, Kizilirmak D, Akgedik R, Bilgi M (2017) Serum levels of magnesium and their relationship with CRP in patients with OSA. Sleep Breath 21(2):549–556. https://doi.org/10.1007/s11325-016-1402-4
Lira AB, de Sousa Rodrigues CF (2016) Evaluation of oxidative stress markers in obstructive sleep apnea syndrome and additional antioxidant therapy: a review article. Sleep Breath 20(4):1155–1160. https://doi.org/10.1007/s11325-016-1367-3
Zhang X, Liu J, Pang X, Zhao J, Wang S, Wu D (2014) Aldosterone induces C-reactive protein expression via MR-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Mol Cell Endocrinol 395(1–2):61–68. https://doi.org/10.1016/j.mce.2014.08.003
Zhao J, Liu J, Pang X, Wang S, Wu D, Zhang X, Feng L (2013) Angiotensin II induces C-reactive protein expression via AT1-ROS-MAPK-NF-kappaB signal pathway in hepatocytes. Cell Physiol Biochem 32(3):569–580. https://doi.org/10.1159/000354461
Funding
This study was supported by the National Natural Science Foundation (No. 81601988).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All human studies have been approved by the Ethical Committee of the Nanjing Medical University and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Significance
We demonstrate that elevated Romo1 levels are almost universal in a cohort with obstructive sleep apnea syndrome (OSAS). Further, we observed significant, independent, positive relationships between Romo1 levels and both OSAS severity, ROS, and CRP. We also found that nCPAP treatment can effectively decrease serum Romo1 concentrations. Future investigation should focus on further clarification of the roles of Romo1 in the pathogenesis of OSAS.
Rights and permissions
About this article
Cite this article
Ye, L., Qian, Y., Li, Q. et al. Serum Romo1 is significantly associated with disease severity in patients with obstructive sleep apnea syndrome. Sleep Breath 22, 743–748 (2018). https://doi.org/10.1007/s11325-017-1606-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1606-2