Abstract
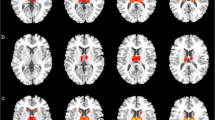
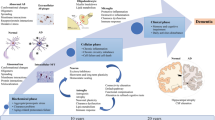
Prion diseases are rare, rapidly progressive, and fatal incurable degenerative brain disorders caused by the misfolding of a normal protein called PrPC into an abnormal protein called PrPSc. Their highly variable clinical presentation mimics various degenerative and non-degenerative brain disorders, making diagnosis a significant challenge for neurologists. Currently, definitive diagnosis relies on post-mortem examination of nervous tissue to detect the pathogenic prion protein. The current diagnostic criteria are limited. While structural magnetic resonance imaging (MRI) remains the gold standard imaging modality for Creutzfeldt-Jakob disease (CJD) diagnosis, positron emission tomography (PET) using 18fluorine-fluorodeoxyglucose (18F-FDG) and other radiotracers have demonstrated promising potential in the diagnostic assessment of prion disease. In this context, a comprehensive and updated review exclusively focused on PET imaging in prion diseases is still lacking. We review the current value of PET imaging with 18F-FDG and non-FDG tracers in the diagnostic management of prion diseases. From the collected data, 18F-FDG PET mainly reveals cortical and subcortical hypometabolic areas in prion disease, although fails to identify typical pattern or laterality abnormalities to differentiate between genetic and sporadic prion diseases. Although the rarity of prion diseases limits the establishment of a definitive hypometabolism pattern, this review reveals some more prevalent 18F-FDG patterns associated with each disease subtype. Interestingly, in both sporadic and genetic prion diseases, the hippocampus does not show significant glucose metabolism alterations, appearing as a useful sign in the differential diagnosis with other neurodegenerative disease. In genetic prion disease forms, PET abnormality precedes clinical manifestation. Discordant diagnostic value for amyloid tracers among different prion disease subtypes was observed, needing further investigation. PET has emerged as a potential valuable tool in the diagnostic armamentarium for CJD. Its ability to visualize functional and metabolic brain changes provides complementary information to structural MRI, aiding in the early detection and confirmation of CJD.


Similar content being viewed by others
References
Uttley L, Carroll C, Wong R, Hilton DA, Stevenson M (2020) Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect Dis 20:e2–e10
Iwasaki Y (2017) Creutzfeldt-Jakob disease. Neuropathology 37:174–188
Arbizu J, Giuliani A, Gallego Perez-Larraya J et al (2017) Emerging clinical issues and multivariate analyses in PET investigations. Q J Nucl Med Mol Imaging 61:386–404
Collins SJ, Sanchez-Juan P, Masters CL et al (2006) Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain 129:2278–2287
Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P (2012) Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 11:618–628
Parchi P, Petersen RB, Chen SG et al (1998) Molecular pathology of fatal familial insomnia. Brain Pathol 8:539–548
Geschwind MD (2015) Prion diseases. Continuum (Minneap Minn) 21:1612–1638
Jeong BH, Kim YS (2014) Genetic studies in human prion diseases. J Korean Med Sci 29:623–632
Zhang Y, Minoshima S, Vesselle H, Lewis DH (2012) A case of Creutzfeldt-Jakob disease mimicking corticobasal degeneration: FDG PET, SPECT, and MRI findings. Clin Nucl Med 37:e173-175
Saint-Aubert L, Pariente J, Dumas H et al (2016) Case report of Lewy body disease mimicking Creutzfeldt-Jakob disease in a 44-year-old man. BMC Neurol 16:122
Miyazawa N (2017) Creutzfeldt-Jakob disease mimicking Alzheimer disease and dementia with Lewy bodies-findings of FDG PET with 3-dimensional stereotactic surface projection. Clin Nucl Med 42:e247–e248
Brown K, Mastrianni JA (2010) The prion diseases. J Geriatr Psychiatry Neurol 23:277–298
Zerr I, Kallenberg K, Summers DM et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132:2659–2668
Chatzikonstantinou S, Kazis D, Karantali E et al (2021) A meta-analysis on RT-QuIC for the diagnosis of sporadic CJD. Acta Neurol Belg 121:341–349
Jones M, Odunsi S, du Plessis D et al (2014) Gerstmann-Straussler-Scheinker disease: novel PRNP mutation and VGKC-complex antibodies. Neurology 82:2107–2111
Hermann P, Appleby B, Brandel JP et al (2021) Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol 20:235–246
Eisenmenger L, Porter MC, Carswell CJ et al (2016) Evolution of diffusion-weighted magnetic resonance imaging signal abnormality in sporadic Creutzfeldt-Jakob disease, with histopathological correlation. JAMA Neurol 73:76–84
Minoshima S, Cross D, Thientunyakit T, Foster NL, Drzezga A (2022) (18)F-FDG PET imaging in neurodegenerative dementing disorders: insights into subtype classification, emerging disease categories, and mixed dementia with copathologies. J Nucl Med 63:2S-12S
Lu H, Jing D, Chen Y et al (2020) Metabolic changes detected by 18F-FDG PET in the preclinical stage of familial Creutzfeldt-Jakob disease. J Alzheimers Dis 77:1513–1521
Qi C, Zhang JT, Zhao W, Xing XW, Yu SY (2020) Sporadic Creutzfeldt-Jakob disease: a retrospective analysis of 104 cases. Eur Neurol 83:65–72
Cortelli P, Perani D, Montagna P et al (2006) Pre-symptomatic diagnosis in fatal familial insomnia: serial neurophysiological and 18FDG-PET studies. Brain 129:668–675
Krasnianski A, Bartl M, Sanchez Juan PJ et al (2008) Fatal familial insomnia: clinical features and early identification. Ann Neurol 63:658–661
Montagna P, Cortelli P, Avoni P et al (1998) Clinical features of fatal familial insomnia: phenotypic variability in relation to a polymorphism at codon 129 of the prion protein gene. Brain Pathol 8:515–520
Xing XW, Zhang JT, Zhu F et al (2012) Comparison of diffusion-weighted MRI with 18F-fluorodeoxyglucose-positron emission tomography/CT and electroencephalography in sporadic Creutzfeldt-Jakob disease. J Clin Neurosci 19:1354–1357
Renard D, Vandenberghe R, Collombier L, Kotzki PO, Pouget JP, Boudousq V (2013) Glucose metabolism in nine patients with probable sporadic Creutzfeldt-Jakob disease: FDG-PET study using SPM and individual patient analysis. J Neurol 260:3055–3064
Zhao W, Zhang JT, Xing XW et al (2013) Chinese specific characteristics of sporadic Creutzfeldt-Jakob disease: a retrospective analysis of 57 cases. PLoS ONE 8:e58442
Krasnianski A, Sanchez Juan P, Ponto C et al (2014) A proposal of new diagnostic pathway for fatal familial insomnia. J Neurol Neurosurg Psychiatry 85:654–659
Ortega-Cubero S, Pagola I, Luquin MR et al (2015) Clinical and neuroimaging characteristics of 14 patients with prionopathy: a descriptive study. Neurologia 30:144–152
Abu-Rumeileh S, Redaelli V, Baiardi S et al (2018) Sporadic fatal insomnia in Europe: phenotypic features and diagnostic challenges. Ann Neurol 84:347–360
Chen Y, Xing XW, Zhang JT et al (2016) Autoimmune encephalitis mimicking sporadic Creutzfeldt-Jakob disease: a retrospective study. J Neuroimmunol 295–296:1–8
Cortelli P, Perani D, Parchi P et al (1997) Cerebral metabolism in fatal familial insomnia: relation to duration, neuropathology, and distribution of protease-resistant prion protein. Neurology 49:126–133
Day GS, Gordon BA, Perrin RJ et al (2018) In vivo [(18)F]-AV-1451 tau-PET imaging in sporadic Creutzfeldt-Jakob disease. Neurology 90:e896–e906
Deters KD, Risacher SL, Yoder KK et al (2016) [(11)C]PiB PET in Gerstmann-Straussler-Scheinker disease. Am J Nucl Med Mol Imaging 6:84–93
Engler H, Lundberg PO, Ekbom K et al (2003) Multitracer study with positron emission tomography in Creutzfeldt-Jakob disease. Eur J Nucl Med Mol Imaging 30:85–95
Hamaguchi T, Kitamoto T, Sato T et al (2005) Clinical diagnosis of MM2-type sporadic Creutzfeldt-Jakob disease. Neurology 64:643–648
Henkel K, Zerr I, Hertel A et al (2002) Positron emission tomography with [(18)F]FDG in the diagnosis of Creutzfeldt-Jakob disease (CJD). J Neurol 249:699–705
Kim EJ, Cho SS, Jeong BH et al (2012) Glucose metabolism in sporadic Creutzfeldt-Jakob disease: a statistical parametric mapping analysis of (18) F-FDG PET. Eur J Neurol 19:488–493
Mente KP, O’Donnell JK, Jones SE et al (2017) Fluorodeoxyglucose positron emission tomography (FDG-PET) correlation of histopathology and MRI in prion disease. Alzheimer Dis Assoc Disord 31:1–7
Okamura N, Shiga Y, Furumoto S et al (2010) In vivo detection of prion amyloid plaques using [(11)C]BF-227 PET. Eur J Nucl Med Mol Imaging 37:934–941
Prieto E, Dominguez-Prado I, Riverol M et al (2015) Metabolic patterns in prion diseases: an FDG PET voxel-based analysis. Eur J Nucl Med Mol Imaging 42:1522–1529
Renard D, Castelnovo G, Collombier L, Thouvenot E, Boudousq V (2017) FDG-PET in Creutzfeldt-Jakob disease: analysis of clinical-PET correlation. Prion 11:440–453
Szpak GM, Lewandowska E, Lechowicz W et al (2006) The brain immune response in human prion diseases. Microglial activation and microglial disease. I Sporadic Creutzfeldt-Jakob Disease Folia Neuropathol 44:202–213
Kaneko M, Sugiyama N, Sasayama D et al (2008) Prion disease causes less severe lesions in human hippocampus than other parts of brain. Psychiatry Clin Neurosci 62:264–270
Anand KS, Dhikav V (2012) Hippocampus in health and disease: an overview. Ann Indian Acad Neurol 15:239–246
Collins S, McLean CA, Masters CL (2001) Gerstmann-Straussler-Scheinker syndrome, fatal familial insomnia, and kuru: a review of these less common human transmissible spongiform encephalopathies. J Clin Neurosci 8:387–397
DeArmond SJ, Prusiner SB (2002) Prion diseases. In: Graham DI, Lantos PL (eds) Greenfield’s neuropathology, 7th ed. Arnold, London, pp 273–323
Kepe V, Ghetti B, Farlow MR et al (2010) PET of brain prion protein amyloid in Gerstmann-Straussler-Scheinker disease. Brain Pathol 20:419–430
Parchi P, Giese A, Capellari S et al (1999) Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46(2):224–233
Ukisu R, Kushihashi T, Kitanosono T et al (2005) Serial diffusion-weighted MRI of Creutzfeldt-Jakob disease. AJR Am J Roentgenol 184:560–566
Ukisu R, Kushihashi T, Tanaka E et al (2006) Diffusion-weighted MR imaging of early-stage Creutzfeldt-Jakob disease: typical and atypical manifestations. Radiographics 26(1):S191-204
Author information
Authors and Affiliations
Contributions
All authors contribute equally to the conception, drafting, and revising the work.
Corresponding author
Ethics declarations
Informed Consent
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria Vittoria Mattoli and Romina Grazia Giancipoli are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mattoli, M.V., Giancipoli, R.G., Cocciolillo, F. et al. The Role of PET Imaging in Patients with Prion Disease: A Literature Review. Mol Imaging Biol 26, 195–212 (2024). https://doi.org/10.1007/s11307-024-01895-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-024-01895-0