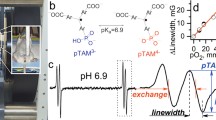
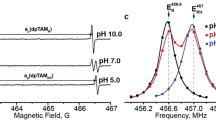
Abstract
Purpose
Hypoxia and acidosis are recognized tumor microenvironment (TME) biomarkers of cancer progression. Alterations in cancer redox status and metabolism are also associated with elevated levels of intracellular glutathione (GSH) and interstitial inorganic phosphate (Pi). This study aims to evaluate the capability of these biomarkers to discriminate between stages and inform on a switch to malignancy.
Procedures
These studies were performed using MMTV-PyMT( +) female transgenic mice that spontaneously develop breast cancer and emulate human tumor staging. In vivo assessment of oxygen concentration (pO2), extracellular acidity (pHe), Pi, and GSH was performed using L-band electron paramagnetic resonance spectroscopy and multifunctional trityl and GSH-sensitive nitroxide probes.
Results
Profiling of the TME showed significant deviation of measured biomarkers upon tumor progression from pre-malignancy (pre-S4) to the malignant stage (S4). For the combined marker, HOP: (pHe × pO2)/Pi, a value > 186 indicated that the tumors were pre-malignant in 85% of the mammary glands analyzed, and when < 186, they were malignant 42% of the time. For GSH, a value < 3 mM indicated that the tumors were pre-malignant 74% of the time, and when > 3 mM, they were malignant 80% of the time. The only marker that markedly deviated as early as stage 1 (S1) from its value in pre-S1 was elevated Pi, followed by a decrease of pHe and pO2 and increase in GSH at later stages.
Conclusion
Molecular TME profiling informs on alteration of tumor redox and metabolism during tumor staging. Early elevation of interstitial Pi at S1 may reflect tumor metabolic alterations that demand elevated phosphorus supply in accordance with the high rate growth hypothesis. These metabolic changes are supported by the following decrease of pHe due to a high tumor reliance on glycolysis and increase of intracellular GSH, a major intracellular redox buffer. The appreciable decrease in TME pO2 was observed only at malignant S4, apparently as a consequence of tumor mass growth and corresponding decrease in perfusion efficacy and increase in oxygen consumption as the tumor cells proliferate.

Reproduced from reference [15] with permission of Nature Publishing Group.





Similar content being viewed by others
References
Siemann DW (2011) Tumor microenvironment. John Wiley & Sons, Ltd., Chichester, UK; Hoboken, NJ, USA, p 436
Tatum JL, Kelloff GJ, Gillies RJ et al (2006) Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 82(10):699–757
Brahimi-Horn MC, Chiche J, Pouyssegur J (2007) Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol 19(2):223–229
Haulica A, Ababei L (1974) Comparative study of glycolytic activity in the erythrocytes of animals with chronic experimental hypoxia and with tumours. Neoplasma 21(1):29–35
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314. https://doi.org/10.1126/science.123.3191.309
Matsumoto K, Hyodo F, Matsumoto A et al (2006) High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res 12(8):2455–2462
Estrela JM, Ortega A, Obrador E (2006) Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 43(2):143–181
Voegtlin C, Thompson JW (1926) Glutathione content of tumor animals. J Biol Chem 70:801–806
Huang Z, Komninou D, Kleinman W et al (2007) Enhanced levels of glutathione and protein glutathiolation in rat tongue epithelium during 4-NQO-induced carcinogenesis. Int J Cancer 120(7):1396–1401
Bobko AA, Eubank TD, Voorhees JL et al (2012) In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magn Reson Med 67:1827–1836
Traverso N, Ricciarelli R, Nitti M et al (2013) Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013:972913
Chang SH, Yu KN, Lee YS et al (2006) Elevated inorganic phosphate stimulates Akt-ERK1/2-Mnk1 signaling in human lung cells. Am J Respir Cell Mol Biol 35(5):528–539
Spina A, Sapio L, Esposito A et al (2013) Inorganic phosphate as a novel signaling molecule with antiproliferative action in MDA-MB-231 breast cancer cells. Biores Open Access 2(1):47–54. https://doi.org/10.1089/biores.2012.0266
Khoshniat S, Bourgine A, Julien M et al (2011) The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell Mol Life Sci 68(2):205–218
Bobko AA, Eubank TD, Driesschaert B et al (2017) Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci Rep 7:41233. https://doi.org/10.1038/srep41233
Lacerda-Abreu MA, Meyer-Fernandes JR (2023) Inorganic phosphate (Pi) in the breast cancer microenvironment: production, transport and signal transduction as potential targets for anticancer strategies. Curr Cancer Drug Tar 23(3):187–198. https://doi.org/10.2174/1568009622666220928140702
Baudelet C, Gallez B (2002) How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med 48(6):980–986. https://doi.org/10.1002/mrm.10318
Colliez F, Safronova MM, Magat J et al (2015) Oxygen mapping within healthy and acutely infarcted brain tissue in humans using the NMR relaxation of lipids: A Proof-Of-Concept Translational Study. PLoS One 10(8):e0135248. https://doi.org/10.1371/journal.pone.0135248
Ojugo AS, McSheehy PM, McIntyre DJ et al (1999) Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed 12(8):495–504
Zhang X, Lin Y, Gillies RJ (2010) Tumor pH and its measurement. J Nucl Med 51(8):1167–1170. https://doi.org/10.2967/jnumed.109.068981
Frenzel T, Kossler S, Bauer H et al (1994) Noninvasive in vivo pH measurements using a fluorinated pH probe and fluorine-19 magnetic resonance spectroscopy. Invest Radiol 29(Suppl 2):S220-222
Aoki Y, Akagi K, Tanaka Y et al (1996) Measurement of intratumor pH by pH indicator used in 19F-magnetic resonance spectroscopy Measurement of extracellular pH decrease caused by hyperthermia combined with hydralazine. Invest Radiol 31(11):680–689
Longo DL, Bartoli A, Consolino L et al (2016) In vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res 76(22):6463–6470. https://doi.org/10.1158/0008-5472.CAN-16-0825
Sheth VR, Li Y, Chen LQ et al (2012) Measuring in vivo tumor pHe with CEST-FISP MRI. Magn Reson Med 67(3):760–768. https://doi.org/10.1002/mrm.23038
Martinez GV, Zhang X, Garcia-Martin ML et al (2011) Imaging the extracellular pH of tumors by MRI after injection of a single cocktail of T1 and T2 contrast agents. NMR Biomed 24(10):1380–1391. https://doi.org/10.1002/nbm.1701
Gallagher FA, Kettunen MI, Day SE et al (2008) Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453(7197):940–943. https://doi.org/10.1038/nature07017
Duwel S, Hundshammer C, Gersch M et al (2017) Imaging of pH in vivo using hyperpolarized (13)C-labelled zymonic acid. Nat Commun 8:15126. https://doi.org/10.1038/ncomms15126
Livesey JC, Golden RN, Shankland EG et al (1992) Magnetic-resonance spectroscopic measurement of cellular thiol reduction-oxidation state. Int J Radiat Oncol 22(4):755–757
Terpstra M, Henry PG, Gruetter R (2003) Measurement of reduced glutathione (GSH) in human brain using LCmodel analysis of difference-edited spectra. Magnet Reson Med 50(1):19–23. https://doi.org/10.1002/mrm.10499
Willis JA, Schleich T (1995) C-13-Nmr spectroscopic studies of 2-mercaptoethanol-stimulated glutathione synthesis in the intact ocular lens. BBA-Mol Cell Res 1265(1):1–7. https://doi.org/10.1016/0167-4889(94)00195-K
Trabesinger AH, Weber OM, Duc CO et al (1999) Detection of glutathione in the human brain in vivo by means of double quantum coherence filtering. Magnet Reson Med 42(2):283–289
Choi C, Zhao C, Dimitrov I et al (2009) Measurement of glutathione in human brain at 3T using an improved double quantum filter in vivo. J Magn Reson 198(2):160–166. https://doi.org/10.1016/j.jmr.2009.02.002
Gillies RJ, Raghunand N, Garcia-Martin ML et al (2004) pH imaging A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag 23(5):57–64
Gade TP, Buchanan IM, Motley MW et al (2009) Imaging intratumoral convection: pressure-dependent enhancement in chemotherapeutic delivery to solid tumors. Clin Cancer Res 15(1):247–255. https://doi.org/10.1158/1078-0432.CCR-08-0611
Khramtsov VV, Bobko AA, Tseytlin M et al (2017) Exchange phenomena in the electron paramagnetic resonance spectra of the nitroxyl and trityl radicals: multifunctional spectroscopy and imaging of local chemical microenvironment. Anal Chem 89(9):4758–4771
Bobko AA, Eubank TD, Driesschaert B et al (2018) In vivo EPR assessment of pH, pO(2), redox status, and concentrations of phosphate and glutathione in the tumor microenvironment. J Vis Exp 133:e56624. https://doi.org/10.3791/56624
Golman K, Petersson JS, Ardenkjaer-Larsen JH et al (2000) Dynamic in vivo oxymetry using overhauser enhanced MR imaging. J Magn Reson Imaging 12:929–938
Ardenkjaer-Larsen JH, Laursen I, Leunbach I et al (1998) EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson 133(1):1–12
Elas M, Williams BB, Parasca A et al (2003) Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magn Reson Med 49(4):682–691
Krishna MC, English S, Yamada K et al (2002) Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A 99(4):2216–2221
Dhimitruka I, Bobko AA, Eubank TD et al (2013) Phosphonated trityl probe for concurrent in vivo tissue oxygen and pH monitoring using EPR-based techniques. JACS 135:5904–5910
Bobko AA, Dhimitruka I, Zweier JL et al (2014) Fourier transform EPR of trityl radicals for multifunctional assessment of chemical microenvironment. Angew Chem Int Edit 53:2735–2738
Song YG, Liu YP, Liu WB et al (2014) Characterization of the binding of the Finland trityl radical with bovine serum albumin. Rsc Adv 4(88):47649–47656. https://doi.org/10.1039/c4ra04616a
Ouari O, Gigmes D (2011) Nitroxides. Royal Society of Chemistry, Croydon, UK, p 592
Khramtsov VV, Yelinova VI, Weiner LM et al (1989) Quantitative determination of SH groups in low- and high-molecular-weight compounds by an electron spin resonance method. Anal Biochem 182(1):58–63. https://doi.org/10.1016/0003-2697(89)90718-5
Khramtsov VV, Yelinova VI, Glazachev YuI et al (1997) Quantitative determination and reversible modification of thiols using imidazolidine biradical disulfide label. J Biochem Biophys Methods 35(2):115–128
Ellman GL (1958) A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys 74(2):443–450. https://doi.org/10.1016/0003-9861(58)90014-6
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Roshchupkina GI, Bobko AA, Bratasz A et al (2008) In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical probe. Free Rad Biol Med 45:312–320
Samouilov A, Efimova OV, Bobko AA et al (2014) In vivo proton-electron double-resonance imaging of extracellular tumor pH using an advanced nitroxide probe. Analyt Chem 86(2):1045–1052
Gorodetskii AA, Eubank TD, Driesschaert B et al (2019) Development of multifunctional Overhauser-enhanced magnetic resonance imaging for concurrent in vivo mapping of tumor interstitial oxygenation, acidosis and inorganic phosphate concentration. Sci Rep 9(1):12093
Bobko AA, Evans J, Denko NC et al (2017) Concurrent longitudinal EPR monitoring of tissue oxygenation, acidosis, and reducing capacity in mouse xenograft tumor models. Cell Biochem Biophys 75:247–253. https://doi.org/10.1007/s12013-016-0733-x
Lin EY, Jones JG, Li P et al (2003) Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 163(5):2113–2126. https://doi.org/10.1016/S0002-9440(10)63568-7
Gluth TD, Poncelet M, DeVience S et al (2021) Large-scale synthesis of a monophosphonated tetrathiatriarylmethyl spin probe for concurrent in vivo measurement of pO(2), pH and inorganic phosphate by EPR. RSC Adv 11(42):25951–25954. https://doi.org/10.1039/d1ra04551b
pTAM fitting app 1.1 (2022) https://github.com/tdg0013/pTAMFittingApp/releases
Monaghan TF, Rahman SN, Agudelo CW et al (2021) Foundational statistical principles in medical research: sensitivity, specificity, positive predictive value, and negative predictive value. Medicina (Kaunas) 57(5):503. https://doi.org/10.3390/medicina57050503
Guy CT, Cardiff RD, Muller WJ (1992) Induction of mammary-tumors by expression of polyomavirus middle T-oncogene - a transgenic mouse model for metastatic disease. Mol Cell Biol 12(3):954–961. https://doi.org/10.1128/Mcb.12.3.954
Elser JJ, Kyle MM, Smith MS et al (2007) Biological stoichiometry in human cancer. PLoS On. 2(10):e0128. https://doi.org/10.1371/journal.pone.0001028
Kareva I (2013) Biological stoichiometry in tumor micro-environments. PLoS One 8(1):51844. https://doi.org/10.1371/journal.pone.0051844
Balendiran GK, Dabur R, Fraser D (2004) The role of glutathione in cancer. Cell Biochem Funct 22(6):343–352. https://doi.org/10.1002/cbf.1149
Khramtsov VV, Gillies RJ (2014) Janus-faced tumor microenvironment and redox. Antioxid Redox Signal 21:723–729
Bi QC, Luo RG, Li YS et al (2022) Low inorganic phosphate stress inhibits liver cancer progression: from In Vivo to In Vitro. Adv Therapeutics 5(2):2100224. https://doi.org/10.1002/adtp.202100224
Falchini GE, Malezan A, Poletti ME et al (2021) Analysis of phosphorous content in cancer tissue by synchrotron micro-XRF. Radiat Phys Chem 179:109157. https://doi.org/10.1016/j.radphyschem.2020.109157
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30(11):1191–1212
Pastore A, Federici G, Bertini E et al (2003) Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 333(1):19–39
Kang YJ, Enger MD (1990) Glutathione content and growth in A549 human lung carcinoma cells. Exp Cell Res 187(1):177–179
Hall AG (1999) Review: the role of glutathione in the regulation of apoptosis. Eur J Clin Invest 29(3):238–245
Ketterer B (1988) Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res 202(2):343–361
Mitchell JB, Biaglow JE, Russo A (1988) Role of glutathione and other endogenous thiols in radiation protection. Pharmacol Ther 39(1–3):269–274
Ward DN, Griffin AC (1955) Phosphorus incorporation into nucleic acids and proteins of liver nuclei of normal and azo dye-fed rats. Can Res 15(7):456–461
Kuang Y, Nagy JD, Elser JJ (2004) Biological stoichiometry of tumor dynamics: mathematical models and analysis. Discrete Cont Dyn-B 4(1):221–240
Papaloucas CD, Papaloucas MD, Kouloulias V et al (2014) Measurement of blood phosphorus: a quick and inexpensive method for detection of the existence of cancer in the body. Too good to be true, or forgotten knowledge of the past? Med Hypotheses 82(1): 24–25. https://doi.org/10.1016/j.mehy.2013.10.028
Wilson KM, Shui IM, Mucci LA et al (2015) Calcium and phosphorus intake and prostate cancer risk: a 24-y follow-up study. Am J Clin Nutr 101(1):173–183. https://doi.org/10.3945/ajcn.114.088716
Jin H, Xu CX, Lim HT et al (2009) High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am J Respir Crit Care Med 179(1):59–68
Camalier CE, Young MR, Bobe G et al (2010) Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev Res (Phila) 3(3):359–370. https://doi.org/10.1158/1940-6207.CAPR-09-0068
Taguchi A, DeVience S, Driesschaert B et al (2020) In vitro simultaneous mapping of the partial pressure of oxygen, pH and inorganic phosphate using electron paramagnetic resonance. Analyst 145(9):3236–3244. https://doi.org/10.1039/d0an00168f
Legenzov EA, Sims SJ, Dirda NDA et al (2015) Disulfide-linked dinitroxides for monitoring cellular thiol redox status through electron paramagnetic resonance spectroscopy. Biochemistry-Us 54(47):6973–6982. https://doi.org/10.1021/acs.biochem.5b00531
Elajaili H, Biller JR, Rosen GM et al (2015) Imaging disulfide dinitroxides at 250 MHz to monitor thiol redox status. J Magn Reson 260:77–82. https://doi.org/10.1016/j.jmr.2015.08.027
Epel B, Sundramoorthy SV, Krzykawska-Serda M et al (2017) Imaging thiol redox status in murine tumors in vivo with rapid-scan electron paramagnetic resonance. J Magn Reson 276:31–36
Gluth TD, Poncelet M, Gencheva M et al (2022) Biocompatible monophosphonated trityl spin probe, HOPE71, for in vivo measurement of pO(2), pH, and [P-I] by electron paramagnetic resonance spectroscopy. Anal Chem. https://doi.org/10.1021/acs.analchem.2c03476
Poncelet M, Huffman JL, Khramtsov VV et al (2019) Synthesis of hydroxyethyl tetrathiatriarylmethyl radicals OX063 and OX071. RSC Adv 9(60):35073–35076. https://doi.org/10.1039/c9ra08633a
Funding
This work was partially supported by NIH grants CA194013, CA192064, EB028553, EB023990, and U54GM104942.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eubank, T.D., Bobko, A.A., Hoblitzell, E.H. et al. In Vivo Electron Paramagnetic Resonance Molecular Profiling of Tumor Microenvironment upon Tumor Progression to Malignancy in an Animal Model of Breast Cancer. Mol Imaging Biol (2023). https://doi.org/10.1007/s11307-023-01847-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11307-023-01847-0