Abstract
Purpose
To provide reliable and reproducible heart/mediastinum (H/M) ratio cut-off values for parkinsonian disorders using two machine learning techniques, Support Vector Machines (SVM) and Random Forest (RF) classifier, applied to [123I]MIBG cardiac scintigraphy.
Procedures
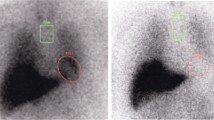
We studied 85 subjects, 50 with idiopathic Parkinson’s disease, 26 with atypical Parkinsonian syndromes (P), and 9 with essential tremor (ET). All patients underwent planar early and delayed cardiac scintigraphy after [123I]MIBG (111 MBq) intravenous injection. Images were evaluated both qualitatively and quantitatively; the latter by the early and delayed H/M ratio obtained from regions of interest (ROIt1 and ROIt2) drawn on planar images. SVM and RF classifiers were finally used to obtain the correct cut-off value.
Results
SVM and RF produced excellent classification performances: SVM classifier achieved perfect classification and RF also attained very good accuracy. The better cut-off for H/M value was 1.55 since it remains the same for both ROIt1 and ROIt2. This value allowed to correctly classify PD from P and ET: patients with H/M ratio less than 1.55 were classified as PD while those with values higher than 1.55 were considered as affected by parkinsonism and/or ET. No difference was found when early or late H/M ratio were considered separately thus suggesting that a single early evaluation could be sufficient to obtain the final diagnosis.
Conclusions
Our results evidenced that the use of SVM and CT permitted to define the better cut-off value for H/M ratios both in early and in delayed phase thus underlining the role of [123I]MIBG cardiac scintigraphy and the effectiveness of H/M ratio in differentiating PD from other parkinsonism or ET. Moreover, early scans alone could be used for a reliable diagnosis since no difference was found between early and late. Definitely, a larger series of cases is needed to confirm this data.


Similar content being viewed by others
References
Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, Brooks D, Burn DJ, Colosimo C, Fanciulli A, Ferreira J, Gasser T, Grandas F, Kanovsky P, Kostic V, Kulisevsky J, Oertel W, Poewe W, Reese JP, Relja M, Ruzicka E, Schrag A, Seppi K, Taba P, Vidailhet M (2013) EFNS/MDS-ES recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol 20:16–34
Postuma RB, Poewe W, Litvan I, Lewis S, Lang AE, Halliday G, Goetz CG, Chan P, Slow E, Seppi K, Schaffer E, Rios-Romenets S, Mi T, Maetzler C, Li Y, Heim B, Bledsoe IO, Berg D (2018) Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 33(10):1601–1608
Hustad E, Skogholt AH, Hveem K, Aasly JO (2018) The accuracy of the clinical diagnosis of Parkinson disease. The HUNT study. J Neurol 265:2120–2124. https://doi.org/10.1007/s00415-018-8969-6
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson’s disease. A systematic review and meta-analysis. Neurology 86:566–576
Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, Sabbagh MN, Sue LI, Jacobson SA, Belden CM, Dugger BN (2014) Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 83:406–412
Shimizu S, Hirao K, Kanetaka H, Namioka N, Hatanaka H, Hirose D, Fukasawa R, Umahara T, Sakurai H, Hanyu H (2016) Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43:184–192
Pahwa R, Lyons KE (2010) Early diagnosis of Parkinson’s disease: recommendations from diagnostic clinical guidelines. Am J Manag Care 16(Suppl):S94–S99
Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Köllensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W (2013) European multiple system atrophy study group. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12:264–274
Wijemanne S, Jankovic J (2015) Dopa-responsive dystonia: clinical and genetic heterogeneity. Nat Rev Neurol 11:414–424
Galpern WR, Corrigan-Curay J, Lang AE (2012) Sham neurosurgical procedures in clinical trials for neurodegenerative diseases: scientific and ethical considerations. Lancet Neurol 11:643–650
Wager TD, Atlas LY (2015) The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 16:403–418
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912
Contrafatto D, Mostile G, Nicoletti A, Dibilio V, Raciti L, Lanzafame S, Luca A, Distefano A, Zappia M (2012) [123I]FP-CIT-SPECT asymmetry index to differentiate Parkinson’s disease from vascular parkinsonism. Acta Neurol Scand 126:12–16
Kagi G, Bhatia KP, Tolosa E (2010) The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry 81:5–12
Zijlmans JC (2010) The role of imaging in the diagnosis of vascular parkinsonism. Neuroimaging Clin N Am 20:69–76
Palumbo B, Fravolini ML, Nuvoli S, Spanu A, Paulus KS, Schillaci O, Madeddu G (2010) Comparison of two neural network classifiers in the differential diagnosis of essential tremor and Parkinson’s disease by 123I-FP-CIT brain SPECT. Eur J Nucl Med Mol Imaging 37:2146–2153
Tolosa E, Borght TV, Moreno E (2007) Accuracy of DaTSCAN (123IIoflupane) SPECT in diagnosis of patients with clinically uncertain parkinsonism: 2-year follow-up of an open-label study. Mov Disord 22:2346–2351
Suwijn SR, van Boheemen CJ, de Haan RJ et al (2015) The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson’s disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res 5:1–8
Gupta D, Kuruvilla A (2011) Vascular parkinsonism: what makes it different? Postgrad Med J 87:829–836
Vallabhajosula S, Nikolopoulou A (2011) Radioiodinated metaiodobenzylguanidine (MIBG): radiochemistry, biology, and pharmacology. Semin Nucl Med 41:324–333
Yamashina S, Yamazaki J (2007) Neuronal imaging using SPECT. Eur J Nucl Med Mol Imaging 34:939–950
Nuvoli S, Spanu A, Piras MR, Nieddu A, Mulas A, Rocchitta G, Galleri G, Serra PA, Madeddu G (2017) 123I-ioflupane brain SPECT and 123I-MIBG cardiac planar scintigraphy combined use in uncertain parkinsonian disorders. Medicine (Baltimore) 96:e6967
Flotats A, Carrió I, Agostini D, le Guludec D, Marcassa C, Schäfers M, Somsen GA, Unlu M, Verberne HJ, EANM Cardiovascular Committee, European Council of Nuclear Cardiology (2010) EANM Cardiovascular Committee; European Council of Nuclear Cardiology. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging 37:1802–1812
Martins da Silva MI, Vidigal Ferreira MJ, Morão Moreirac AP (2013) Iodine-123-metaiodobenzylguanidine scintigraphy in risk stratification of sudden death in heart failure. Rev Port Cardiol 32:509–516
Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BLF (2008) Prognostic value of myocardial 123Imetaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J 29:1147–1159
Oka H, Toyoda C, Yogo M, Mochio S (2011) Reduced cardiac 123I-MIBG uptake reflects cardiac sympathetic dysfunction in de novo Parkinson’s disease. J Neural Transm 118:1323–1327
Orimo S, Suzuki M, Inaba A, Mizusawa H (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord 18:494–500
Shin DH, Lee PH, Bang OY, Joo IS, Huh K (2006) Clinical implications of cardiac-MIBG SPECT in the differentiation of parkinsonian syndromes. J Clin Neurol 2:51–57
Kim JS, Lee PH, Lee KS, Park JW, Kim YI, Chung YA, Kim SH, Kim SH, Kim J, Choi YY, Kim HT (2006) Cardiac [123I]metaiodobenzylguanidine scintigraphy for vascular parkinsonism. Mov Disord 21:1990–1994
Kalra S, Grosset DG, Benamer HTS (2010) Differentiating vascular parkinsonism from idiopathic Parkinson’s disease: a systematic review. Mov Disord 25:149–156
Miyamoto T, Miyamoto M, Suzuki K, Nishibayashi M, Iwanami M, Hirata K (2008) 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep 31:717–723
Kane JPM, Roberts G, Petrides GS, Lloyd JJ, O’Brien JT, Thomas AJ (2019) 123I-MIBG scintigraphy utility and cut-off value in a clinically representative dementia cohort. Parkinsonism Relat Disord 26:79–84. https://doi.org/10.1016/j.parkreldis.2019.01.024
Deuschl G, Bain P, Brin M (1998) Consensus statement of the Movement Disorder Society on tremor. Ad hoc Scientific Committee. Mov Disord 13(Suppl 3):2–23
Hoehn M, Yahr MD (1967) Parkinsonism: onset, progression, and mortality. Neurology 17:427–442
Takatsu H, Nishida H, Matsuo H et al (2000) Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med 41:71–77
Chang CC, Lin CJ (2011) LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol 2:27 Software available at http://www.csie.ntu.edu.tw/~cjlin/libsvm
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20:273–297
Shawe-Taylor J, Bartlett PL (1998) Structural risk minimization over data-dependent hierarchies. IEEE Trans Inf Theory 44:1926–1940
Hastie T, Tibshirani R, Friedman J (2009) The elements of statistical learning, springer series in statistics. Springer New York Inc., New York
Criminisi A, Shotton J, Konukoglu E (2012) Decision forests: a unified framework for classification, regression, density estimation, manifold learning and semi-supervised learning. Foundations and Trends in Computer Graphics and Vision 7:81–227
Nuvoli S, Palumbo B, Malaspina S, Madeddu G, Spanu A (2018) 123I-ioflupane SPET and 123I-MIBG in the diagnosis of Parkinson’s disease and parkinsonian disorders and in the differential diagnosis between Alzheimer’s and Lewy’s bodies dementias. Hell J Nucl Med 21:60–68
Amit Y, Geman D (1997) Shape quantization and recognition with randomized trees. Neural Comput 9:1545–1588
Breiman L, Friedman JH, Olshen RA et al (1984) Classification and regression trees. Chapman and Hall/CRC
Breiman L (2001) Random forests. Mach Learn 45:5–32
Knudsen K, Borghammer P (2018) Imaging the autonomic nervous system in Parkinson’s disease. Curr Neurol Neurosci Rep 18:79
Okada Y, Shiraishi M, Nakamura H, Maki F, Sasaki N, Hasegawa Y, Sasaki O, Nakashima Y (2018) Usefulness of the combination of iodine-123-metaiodobenzylguanidine scintigraphy and iodine-123-ioflupane scintigraphy in new-onset Parkinson’s disease. Nucl Med Commun 39:983–988
Yoshita M (1998) Differentiation of idiopathic Parkinson’s disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci 155:60–67
Südmeyer M, Antke C, Zizek T et al (2011) Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J Nucl Med 52:733–740
Sakamoto F, Shiraishi S, Tsuda N et al (2016) 123I-MIBG myocardial scintigraphy for the evaluation of Lewy body disease: are delayed images essential? Is visual assessment useful? Br J Radiol 10:20160144
Bianconi F, Fravolini ML, Bello-Cerezo R, Minestrini M, Scialpi M, Palumbo B (2018) Evaluation of shape and textural features from CT as prognostic biomarkers in non-small cell lung cancer. Anticancer Res 38:2155–2160
Palumbo B, Fravolini ML, Buresta T, Pompili F, Forini N, Nigro P, Calabresi P, Tambasco N (2014) Diagnostic accuracy of Parkinson disease by support vector machine (SVM) analysis of 123I-FP-CIT brain SPECT data: implications of putaminal findings and age. Medicine (Baltimore) 93:e228. https://doi.org/10.1097/MD.0000000000000228
Towey DJ, Bain PG, Nijran KS (2011) Automatic classification of 123I-FP-CIT (DaTSCAN) SPECT images. Nucl Med Commun 32:699–707
Taylor JC, Fenner JW (2017) Comparison of machine learning and semi-quantification algorithms for (I123)FP-CIT classification: the beginning of the end for semi-quantification? EJNMMI Phys 4:29. https://doi.org/10.1186/s40658-017-0196-1
Castillo-Barnes D, Ramírez J, Segovia F et al (2018) Robust ensemble classification methodology for I123-Ioflupane SPECT images and multiple heterogeneous biomarkers in the diagnosis of Parkinson's disease. Front Neuroinform 12:53. https://doi.org/10.3389/fninf.2018.00053 eCollection 2018
Goldstein DS, Holmes C, Kopin IJ, Sharabi Y (2011) Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest 121:3320–3330
Cascianelli S, Scialpi M, Amici S, Forini N, Minestrini M, Fravolini M, Sinzinger H, Schillaci O, Palumbo B (2017) Role of artificial intelligence techniques (automatic classifiers) in molecular imaging modalities in neurodegenerative diseases. Curr Alzheimer Res 14:198–207
Gray KR, Aljabar P, Heckemann RA, Hammers A, Rueckert D, Alzheimer’s Disease Neuroimaging Initiative (2013) Alzheimer’s disease neuroimaging initiative. Random forest-based similarity measures for multi-modal classification of Alzheimer’s disease. Neuroimage 65:167–175
Segovia F, Górriz JM, Ramírez J et al (2017) Preprocessing of 18F-DMFP-PET data based on hidden Markov random fields and the Gaussian distribution. Front Aging Neurosci 9:326. https://doi.org/10.3389/fnagi.2017.00326 eCollection 2017
Segovia F, Illán IA, Górriz JM et al (2015) Distinguishing Parkinson’s disease from atypical parkinsonian syndromes using PET data and a computer system based on support vector machines and Bayesian networks. Front Comput Neurosci 9:137. https://doi.org/10.3389/fncom.2015.00137 eCollection 2015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nuvoli, S., Spanu, A., Fravolini, M.L. et al. [123I]Metaiodobenzylguanidine (MIBG) Cardiac Scintigraphy and Automated Classification Techniques in Parkinsonian Disorders. Mol Imaging Biol 22, 703–710 (2020). https://doi.org/10.1007/s11307-019-01406-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01406-6