Abstract
Purpose
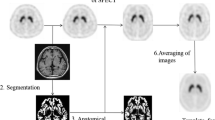
[123I]FP-CIT (DaTSCAN®) single-photon emission computed tomography (SPECT) imaging is widely used to study neurodegenerative parkinsonism, by measuring presynaptic dopamine transporter (DAT) in striatal regions. Beyond DAT, [123I]FP-CIT may be considered for other monoaminergic systems, in particular the serotonin transporter (SERT). Independent component analysis (ICA) implemented in source-based morphometry (SBM) could represent an alternative method to explore monoaminergic pathways, studying the relationship among voxels and grouping them into “neurotransmission” networks.
Procedures
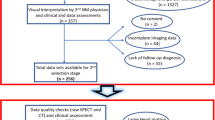
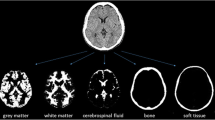
One hundred forty-three subjects [84 with Parkinson’s disease (PD) and 59 control individuals (CG)] underwent DATSCAN® imaging. The [123I]FP-CIT binding was evaluated by multivariate SBM approach, as well as by a whole-brain voxel-wise univariate (statistical parametric mapping, SPM) approach.
Results
As compared to the univariate whole-brain approach (SPM) (only demonstrating striatal [123I]FP-CIT binding reduction in PD group), SBM identified six sources of non-artefactual origin, including basal ganglia and cortical regions as well as brainstem. Among them, three sources (basal ganglia and cortical regions) presented loading scores (as index of [123I]FP-CIT binding) significantly different between PD and CG. Notably, even if not significantly different between PD and CG, the remaining three non-artefactual sources were characterized by a predominant frontal, brainstem, and occipito-temporal involvement.
Conclusion
The concept of source blind separation by the application of ICA (as implemented in SBM) represents a feasible approach to be considered in [123I]FP-CIT (DaTSCAN®) SPECT imaging. Taking advantage of this multivariate analysis, specific patterns of variance can be identified (involving either striatal than extrastriatal regions) that could be useful in differentiating neurodegenerative parkinsonisms.


Similar content being viewed by others
References
Booij J, Tissingh G, Boer GJ et al (1997) [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:133–140
Seibyl JP, Marek K, Sheff K et al (1997) Test/retest reproducibility of iodine-123-betaCIT SPECT brain measurement of dopamine transporters in Parkinson’s patients. J Nucl Med 38:1453–1459
Benamer TS, Patterson J, Grosset DG et al (2000) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord 15:503–510
Lorberboym M, Treves TA, Melamed E et al (2006) [123I]-FP/CIT SPECT imaging for distinguishing drug-induced parkinsonism from Parkinson’s disease. Mov Disord 21:510–514
Brigo F, Matinella A, Erro R, Tinazzi M (2014) [(1)(2)(3)I]FP-CIT SPECT (DaTSCAN) may be a useful tool to differentiate between Parkinson’s disease and vascular or drug-induced parkinsonisms: a meta-analysis. Eur J Neurol 21:1369–e1390
Oh M, Kim JS, Kim JY et al (2012) Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med 53:399–406
Lundkvist C, Halldin C, Swahn CG et al (1995) [O-methyl-11C]beta-CIT-FP, a potential radioligand for quantitation of the dopamine transporter: preparation, autoradiography, metabolite studies, and positron emission tomography examinations. Nucl Med Biol 22:905–913
Geisler S, Beindorff N, Cremer M et al (2015) Characterization of [123I]FP-CIT binding to the dopamine transporter in the striatum of tree shrews by quantitative in vitro autoradiography. Synapse 69:497–504
Catafau AM, Tolosa E (2004) Impact of dopamine transporter SPECT using 123I-ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes. Mov Disord 19:1175–1182
Varrone A, Halldin C (2010) Molecular imaging of the dopamine transporter. J Nucl Med 51:1331–1334
Varrone A, Halldin C (2012) New developments of dopaminergic imaging in Parkinson’s disease. Q J Nucl Med Mol Imaging 56:68–82
Laruelle M, Baldwin RM, Malison RT et al (1993) SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 13:295–309
Kuikka JT, Tiihonen J, Bergstrom KA et al (1995) Imaging of serotonin and dopamine transporters in the living human brain. Eur J Nucl Med 22:346–350
Neumeyer JL, Tamagnan G, Wang S et al (1996) N-substituted analogs of 2 beta-carbomethoxy-3 beta- (4′-iodophenyl)tropane (beta-CIT) with selective affinity to dopamine or serotonin transporters in rat forebrain. J Med Chem 39:543–548
Scheffel U, Lever JR, Abraham P et al (1997) N-substituted phenyltropanes as in vivo binding ligands for rapid imaging studies of the dopamine transporter. Synapse 25:345–349
Okada T, Fujita M, Shimada S et al (1998) Assessment of affinities of beta-CIT, beta-CIT-FE, and beta-CIT-FP for monoamine transporters permanently expressed in cell lines. Nucl Med Biol 25:53–58
Gunther I, Hall H, Halldin C et al (1997) [125I] beta-CIT-FE and [125I] beta-CIT-FP are superior to [125I] beta-CIT for dopamine transporter visualization: autoradiographic evaluation in the human brain. Nucl Med Biol 24:629–634
Kish SJ, Furukawa Y, Chang LJ et al (2005) Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region? Nucl Med Biol 32:123–128
Isaias IU, Marotta G, Pezzoli G et al (2011) Enhanced catecholamine transporter binding in the locus coeruleus of patients with early Parkinson disease. BMC Neurol 11:88
Booij J, de Jong J, de Bruin K et al (2007) Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med 48:359–366
Pencharz DR, Hanlon P, Chakravartty R et al (2014) Automated quantification with BRASS reduces equivocal reporting of DaTSCAN (123I-FP-CIT) SPECT studies. Nucl Med Rev Cent East Eur 17:65–69
Varrone A, Dickson JC, Tossici-Bolt L et al (2013) European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging 40:213–227
Nicastro N, Garibotto V, Poncet A et al (2016) Establishing on-site reference values for (123)I-FP-CIT SPECT (DaTSCAN(R)) using a cohort of individuals with non-degenerative conditions. Mol Imaging Biol 18:302–312
Colloby SJ, O’Brien JT, Fenwick JD et al (2004) The application of statistical parametric mapping to 123I-FP-CIT SPECT in dementia with Lewy bodies, Alzheimer’s disease and Parkinson’s disease. NeuroImage 23:956–966
Kaasinen V, Joutsa J, Noponen T et al (2015) Effects of aging and gender on striatal and extrastriatal [123I]FP-CIT binding in Parkinson’s disease. Neurobiol Aging 36:1757–1763
El Fakhri G, Habert MO, Maksud P et al (2006) Quantitative simultaneous (99 m)Tc-ECD/123I-FP-CIT SPECT in Parkinson’s disease and multiple system atrophy. Eur J Nucl Med Mol Imaging 33:87–92
Premi E, Pilotto A, Garibotto V, et al. (2016) Impulse control disorder in PD: A lateralized monoaminergic frontostriatal disconnection syndrome? Parkinsonism Relat Disord
Frosini D, Unti E, Guidoccio F et al (2015) Mesolimbic dopaminergic dysfunction in Parkinson’s disease depression: evidence from a 123I-FP-CIT SPECT investigation. J Neural Transm (Vienna) 122:1143–1147
Eusebio A, Azulay JP, Ceccaldi M et al (2012) Voxel-based analysis of whole-brain effects of age and gender on dopamine transporter SPECT imaging in healthy subjects. Eur J Nucl Med Mol Imaging 39:1778–1783
Koch W, Unterrainer M, Xiong G et al (2014) Extrastriatal binding of [(1)(2)(3)I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging 41:1938–1946
Margulies DS, Bottger J, Long X et al (2010) Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. Magma 23:289–307
Beckmann CF (2012) Modelling with independent components. NeuroImage 62:891–901
Zeman PM, Till BC, Livingston NJ et al (2007) Independent component analysis and clustering improve signal-to-noise ratio for statistical analysis of event-related potentials. Clin Neurophysiol 118:2591–2604
Xu L, Groth KM, Pearlson G et al (2009) Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp 30:711–724
Caprihan A, Abbott C, Yamamoto J et al (2011) Source-based morphometry analysis of group differences in fractional anisotropy in schizophrenia. Brain Connect 1:133–145
Fornito A, Zalesky A, Breakspear M (2015) The connectomics of brain disorders. Nat Rev Neurosci 16:159–172
Montembeault M, Joubert S, Doyon J et al (2012) The impact of aging on gray matter structural covariance networks. NeuroImage 63:754–759
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Bain P, Brin M, Deuschl G et al (2000) Criteria for the diagnosis of essential tremor. Neurology 54:S7
Garcia-Gomez FJ, Garcia-Solis D, Luis-Simon FJ et al (2013) Elaboration of the SPM template for the standardization of SPECT images with 123I-ioflupane. Rev Esp Med Nucl Imagen Mol 32:350–356
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15:870–878
Steenwijk MD, Geurts JJ, Daams M et al (2016) Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 139:115–126
Eckert MA, Keren NI, Roberts DR et al (2010) Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci 4:10
Gupta CN, Calhoun VD, Rachakonda S et al (2015) Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull 41:1133–1142
Rektorova I, Biundo R, Marecek R et al (2014) Grey matter changes in cognitively impaired Parkinson’s disease patients. PLoS One 9:e85595
Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151
Bell AJ, Sejnowski TJ (1995) An information-maximization approach to blind separation and blind deconvolution. Neural Comput 7:1129–1159
Himberg J, Hyvarinen A, Esposito F (2004) Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage 22:1214–1222
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19:1233–1239
Ziebell M, Holm-Hansen S, Thomsen G et al (2010) Serotonin transporters in dopamine transporter imaging: a head-to-head comparison of dopamine transporter SPECT radioligands 123I-FP-CIT and 123I-PE2I. J Nucl Med 51:1885–1891
Roselli F, Pisciotta NM, Pennelli M et al (2010) Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov Disord 25:1853–1859
Seppi K, Scherfler C, Donnemiller E et al (2006) Topography of dopamine transporter availability in progressive supranuclear palsy: a voxelwise [123I]beta-CIT SPECT analysis. Arch Neurol 63:1154–1160
Davidsson A, Georgiopoulos C, Dizdar N et al (2014) Comparison between visual assessment of dopaminergic degeneration pattern and semi-quantitative ratio calculations in patients with Parkinson’s disease and atypical parkinsonian syndromes using DaTSCAN(R) SPECT. Ann Nucl Med 28:851–859
Walker Z, Moreno E, Thomas A et al (2015) Clinical usefulness of dopamine transporter SPECT imaging with 123I-FP-CIT in patients with possible dementia with Lewy bodies: randomised study. Br J Psychiatry 206:145–152
Berding G, Brucke T, Odin P, et al. (2003) [[123I]beta-CIT SPECT imaging of dopamine and serotonin transporters in Parkinson’s disease and multiple system atrophy. Nuklearmedizin 42:31–38
Scherfler C, Seppi K, Donnemiller E et al (2005) Voxel-wise analysis of [123I]beta-CIT SPECT differentiates the Parkinson variant of multiple system atrophy from idiopathic Parkinson’s disease. Brain 128:1605–1612
Badoud S, Van De Ville D, Nicastro N et al (2016) Discriminating among degenerative parkinsonisms using advanced (123)I-ioflupane SPECT analyses. Neuroimage Clin 12:234–240
Acknowledgements
We would like to thank all participants to the study and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There is no financial interest to report.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the present study.
Rights and permissions
About this article
Cite this article
Premi, E., Calhoun, V.D., Garibotto, V. et al. Source-Based Morphometry Multivariate Approach to Analyze [123I]FP-CIT SPECT Imaging. Mol Imaging Biol 19, 772–778 (2017). https://doi.org/10.1007/s11307-017-1052-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1052-3