Abstract
Purpose
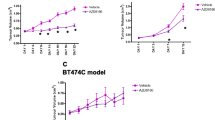
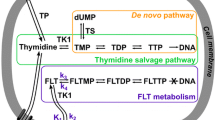
Here, we describe the efficacy of the novel small molecule c-Met inhibitor BAY 853474 in reducing tumor growth in the Hs746T gastric cancer xenograft model and tested the suitability of 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) versus 3′-deoxy-3′-18F-fluorothymidine ([18F]FLT) for response monitoring in a gastric cancer xenograft mouse model using small animal PET.
Procedures
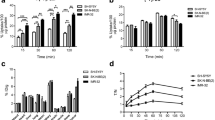
The c-Met inhibitor or vehicle control was administered orally at various doses in tumor-bearing mice. Glucose uptake and proliferation was measured using PET before, 48 and 96 h after the first treatment. The PET data were compared to data from tumor growth curves, autoradiography, Glut-1 and Ki-67 staining of tumor sections, and biochemical analysis of tissue probes, i.e., c-Met and ERK phosphorylation and cyclin D1 levels.
Results
BAY 853474 significantly reduces tumor growth. [18F]FDG uptake in Hs746T tumors was significantly reduced in the groups receiving the drug, compared with the control group. The [18F]FLT uptake in the tumor tissue was completely absent 96 h after treatment. Autoradiographic, immunohistochemical, and biochemical analyses confirmed the PET findings. Treatment with the c-Met inhibitor did not affect body weight or glucose levels, and no adverse effects were observed in the animals.
Conclusion
These preclinical findings suggest that clinical PET imaging is a useful tool for early response monitoring in clinical studies.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Wagner AD, Grothe W, Haerting J et al (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411:355–365
Shaw AT, Yeap BY, Solomon BJ et al (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12:1004–1012
Peschard P, Park M (2007) From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 26:1276–1285
Christensen JG, Burrows J, Salgia R (2005) c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett 225:1–26
Bottaro DP, Rubin JS, Faletto DL et al (1991) Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251:802–804
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4:915–925
Di Renzo MF, Narsimhan RP, Olivero M et al (1991) Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene 6:1997–2003
Liu X, Newton RC, Scherle PA (2010) Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med 16:37–45
Soman NR, Correa P, Ruiz BA, Wogan GN (1991) The TPR-MET oncogenic rearrangement is present and expressed in human gastric carcinoma and precursor lesions. Proc Natl Acad Sci U S A 88:4892–4896
Houldsworth J, Cordon-Cardo C, Ladanyi M, Kelsen DP, Chaganti RS (1990) Gene amplification in gastric and esophageal adenocarcinomas. Cancer Res 50:6417–6422
Kuniyasu H, Yasui W, Kitadai Y et al (1992) Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun 189:227–232
Ott K, Herrmann K, Schuster T et al (2011) Molecular imaging of proliferation and glucose utilization: utility for monitoring response and prognosis after neoadjuvant therapy in locally advanced gastric cancer. Ann Surg Oncol 18:3316–3323
Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–1782
Chen J, Cheong JH, Yun MJ et al (2005) Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 103:2383–2390
Vallbohmer D, Holscher AH, Schneider PM et al (2010) [18F]-fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J Surg Oncol 102:135–140
Shields AF, Grierson JR, Dohmen BM et al (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 4:1334–1336
Kenny LM, Aboagye EO, Price PM (2004) Positron emission tomography imaging of cell proliferation in oncology. Clin Oncol (R Coll Radiol) 16:176–185
Kukuk D, Reischl G, Raguin O et al (2011) Assessment of PET tracer uptake in hormone-independent and hormone-dependent xenograft prostate cancer mouse models. J Nucl Med 52:1654–1663
Fuchs K, Kukuk D, Reischl G et al (2012) Oxygen breathing affects 3′-deoxy-3′-18F-fluorothymidine uptake in mouse models of arthritis and cancer. J Nucl Med 53:823–830
Judenhofer MS, Wehrl HF, Newport DF et al (2008) Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med 14:459–465
Reischl G, Blocher A, Wei R et al (2006) Simplified, automated synthesis of 3′-[18F]fluoro-3′-deoxy-thymidine ([18F]FLT) and simple method for metabolite analysis in plasma. Radiochim Acta 94:447–451
Hamacher K, Coenen HH, Stocklin G (1986) Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 27:235–238
Comoglio PM, Giordano S, Trusolino L (2008) Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 7:504–516
Tseng JR, Kang KW, Dandekar M et al (2008) Preclinical efficacy of the c-Met inhibitor CE-355621 in a U87 MG mouse xenograft model evaluated by 18F-FDG small-animal PET. J Nucl Med 49:129–134
Cullinane C, Dorow DS, Kansara M et al (2005) An in vivo tumor model exploiting metabolic response as a biomarker for targeted drug development. Cancer Res 65:9633–9636
Cullinane C, Dorow DS, Jackson S et al (2011) Differential (18)F-FDG and 3′-deoxy-3′-(18)F-fluorothymidine PET responses to pharmacologic inhibition of the c-MET receptor in preclinical tumor models. J Nucl Med 52:1261–1267
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Brindle K (2008) New approaches for imaging tumour responses to treatment. Nat Rev Cancer 8:94–107
Herrmann K, Ott K, Buck AK et al (2007) Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 48:1945–1950
Kameyama R, Yamamoto Y, Izuishi K et al (2009) Detection of gastric cancer using 18F-FLT PET: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 36:382–388
Mochiki E, Kuwano H, Katoh H et al (2004) Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg 28:247–253
Sampson ER, Martin BA, Morris AE et al (2011) The orally bioavailable met inhibitor PF-2341066 inhibits osteosarcoma growth and osteolysis/matrix production in a xenograft model. J Bone Miner Res 26:1283–1294
Wen PY, Schiff D, Cloughesy TF et al (2011) A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol 13:437–446
Jin H, Yang R, Zheng Z et al (2008) MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res 68:4360–4368
Acknowledgments
We are grateful to Maren Koenig, Mareike Lehnhoff, Daniel Bukala, Nadine Bauer, Denis Lamparter and Monika Klotz, Elke Schmid, Kirsten Steiner-Hahn, Jenny Laube, Tanja Rose for expert technical assistance. The research was partially supported by the Deutsche Forschungsgemeinschaft SFB 773.
Disclosure of Potential Conflicts of Interest
Oliver von Ahsen, Lars Röse, and Andre Mueller are full-time employees of Bayer Healthcare.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 338 kb)
Rights and permissions
About this article
Cite this article
Wiehr, S., von Ahsen, O., Röse, L. et al. Preclinical Evaluation of a Novel c-Met Inhibitor in a Gastric Cancer Xenograft Model Using Small Animal PET. Mol Imaging Biol 15, 203–211 (2013). https://doi.org/10.1007/s11307-012-0580-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-012-0580-0