Abstract
Purpose
The aim of this study is to compare the utility of two positron emission tomography (PET) imaging ligands ((+)-[11C]dihydrotetrabenazine ([11C]DTBZ) and the fluoropropyl analog ([18F]FP-(+)-DTBZ)) that target islet β-cell vesicular monoamine transporter type II to measure pancreatic β-cell mass (BCM).
Procedures
[11C]DTBZ or [18F]FP-(+)-DTBZ was injected, and serial PET images were acquired in rat models of diabetes (streptozotocin-treated and Zucker diabetic fatty) and β-cell compensation (Zucker fatty). Radiotracer standardized uptake values (SUV) were correlated to pancreas insulin content measured biochemically and histomorphometrically.
Results
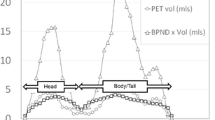
On a group level, a positive correlation of [11C]DTBZ pancreatic SUV with pancreas insulin content and BCM was observed. In the STZ diabetic model, both [18F]FP-(+)-DTBZ and [11C]DTBZ correlated positively with BCM, although only ∼25% of uptake could be attributed to β-cell uptake. [18F]FP-(+)-DTBZ displacement studies indicate that there is a substantial fraction of specific binding that is not to pancreatic islet β cells.
Conclusions
PET imaging with [18F]FP-(+)-DTBZ provides a noninvasive means to quantify insulin-positive BCM and may prove valuable as a diagnostic tool in assessing treatments to maintain or restore BCM.






Similar content being viewed by others
References
Finegood DT, McArthur MD, Kojwang D et al (2001) Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 50:1021–1029
Medarova Z, Evgenov NV, Dai G, Bonner-Weir S, Moore A (2006) In vivo multimodal imaging of transplanted pancreatic islets. Nat Protoc 1:429–435
Saudek F, Brogren CH, Manohar S (2008) Imaging the beta-cell mass: why and how. Rev Diab Stud RDS 5:6–12
Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A (2008) VMAT2 gene expression and function as it applies to imaging beta-cell mass. J Molecul Med 86:5–16
Robertson RP (2007) Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes 56:2420–2424
Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A (2006) In vivo imaging of islet transplantation. Nat Med 12:144–148
Antkowiak PF, Tersey SA, Carter JD, Vandsburger MH, Nadler JL, Epstein FH, Mirmira RG (2009) Noninvasive assessment of pancreatic beta-cell function in vivo with manganese-enhanced magnetic resonance imaging. Am J Physiol Endocrin Metab 296:E573–E578
Schmitz A, Shiue CY, Feng Q et al (2004) Synthesis and evaluation of fluorine-18 labeled glyburide analogs as beta-cell imaging agents. Nucl Med Biol 31:483–491
Schneider S, Feilen PJ, Schreckenberger M et al (2005) In vitro and in vivo evaluation of novel glibenclamide derivatives as imaging agents for the non-invasive assessment of the pancreatic islet cell mass in animals and humans. Exp Clin Endocrinol Diab 113:388–395
Wangler B, Beck C, Shiue CY et al (2004) Synthesis and in vitro evaluation of (S)-2-([I11C]methoxy)-4-[3-methyl-1-(2-piperidine-1-yl-phenyl)-butyl-carbamoyl]-benzoic acid ([11C]methoxy-repaglinide): a potential beta-cell imaging agent. Bioorg Med Chem Lett 14:5205–5209
Wangler B, Schneider S, Thews O et al (2004) Synthesis and evaluation of (S)-2-(2-[18F]fluoroethoxy)-4-([3-methyl-1-(2-piperidin-1-yl-phenyl)-butyl-carbamoyl]-methyl)-benzoic acid ([18F]repaglinide): a promising radioligand for quantification of pancreatic beta-cell mass with positron emission tomography (PET). Nucl Med Biol 31:639–647
Kung HF, Lieberman BP, Zhuang Z-P et al (2008) In vivo imaging of vesicular monoamine transporter 2 in pancreas using an 18F epoxide derivative of tetrabenazine. Nucl Med Biol 35:825–837
Kung M-P, Hou C, Lieberman BP et al (2008) In vivo imaging of β-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J Nucl Med 49:1171–1176
Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A (2008) VMAT2 gene expression and function as it applies to imaging beta-cell mass. J Molec Med 86:5–16
Simpson NR, Souza F, Witkowski P et al (2006) Visualizing pancreatic β-cell mass with [11C]DTBZ. Nuc Med Biol 33:855–864
Souza F, Simpson N, Raffo A et al (2006) Longitudinal noninvasive PET-based β-cell mass estimates in a spontaneous diabetes rat model. J Clin Invest 116:1506–1513
Goland R, Freeby M, Parsey R et al (2009) 11C-Dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med 50:382–389
Weihe E, Eiden LE (2000) Chemical neuroanatomy of the vesicular amine transporters. FASEB J 14:2435–2449
Anlauf MR, Eissele MK, Schafer LE et al (2003) Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J Histochem Cytochem 51:1027–1040
Maffei AZ, Liu P, Witkowski F et al (2004) Identification of tissue-restricted transcripts in human islets. Endocrin 145:4513–4521
Weihe E, Schafer MK, Erickson JD, Eiden LE (1994) Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Molec Neurosci MN 5:149–164
Kung MP, Hou C, Goswami R, Ponde DE, Kilbourn MR, Kung HF (2007) Characterization of optically resolved 9-fluoropropyl-dihydrotetrabenazine as a potential PET imaging agent targeting vesicular monoamine transporters. Nucl Med Biol 34:239–246
Kilbourn MR, Hockleya B, Leea L et al (2007) Pharmacokinetics of [18F]fluoroalkyl derivatives of dihydrotetrabenazine in rat and monkey brain. Nuc Med Biol 34:233–237
Larsen P, Ulin J, Dahlstrom K, Jensen M (1997) Synthesis of [11C]iodomethane by iodination of [11C]methane. Appl Radiat Isot 48:153–157
Jewett D (1992) A simple synthesis of [11C]methyl triflate. Appl Radiat Isot 43:1383–1385
Liu YQ, Jetton TL, Leahy JL (2002) β-Cell adaptation to insulin resistance. J Biol Chem 277:39163–39168
Pick A, Kubstrub CJ, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS (1998) Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the Zucker diabetic fatty rat. Diabetes 47:358–364
Carson RE, Barker WC, Liow JS, Johnson CA (2003) Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. IEEE Nucl Sci Symp Conf Rec 5:3281–3285
Rasband WS. Image J. U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2009
Brenna O, Qvigstad G, Brenna E, Waldum HL (2003) Cytotoxicity of streptozotocin on neuroendocrine cells of the pancreas and the gut. Dig Dis Sci 48:906–910
Takahashi T, Kantoh M, Kusunoki M, Yamamura T, Utsunomiya J (1989) Different innervation mechanisms between the lesser and greater curvature of guinea pig antrum. Dig Dis Sci 34:220–224
Saisho Y, Harris PE, Butler AE, Galasso R, Gurlo T, Rizza RA, Butler PC (2008) Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol Hist 39:543–551
Ichise M, Liow JS et al (2003) Linearized reference tissue parametricimaging methods: application to [11C]DASB positron emission tomographystudies of the serotonin transporter in human brain. J Cereb Blood FlowMetab 23:1096–1112
Bray GA (1977) The Zucker-fatty rat: a review. Fed Proc 36:148–153
Ohneda M, Inman LR, Unger RH (1995) Caloric restriction in obese pre-diabetic rats prevents beta-cell depletion, loss of beta-cell GLUT 2 and glucose incompetence. Diabetologia 38:173–179
Clark JB, Palmer CJ, Shaw WN (1983) The diabetic Zucker fatty rat. Proc Soc Exp Biol Med 173:68–75
Janssen SW, Hermus AR, Lange WP (2001) Progressive histopathological changes in pancreatic islets of Zucker diabetic fatty rats. Exp Clinic Endoc Diab 109:273–282
Mei Q, Mundinger TO, Lernmark A, Taborsky GJ Jr (2002) Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 51:2997–3002
Raffo A, Hancock K, Polito T et al (2008) Role of vesicular monoamine transporter type 2 in rodent insulin secretion and glucose metabolism revealed by its specific antagonist tetrabenazine. J Endocrin 198:41–49
Lenzen S (2008) The mechanism of alloxan- and streptozotocin-induced diabetes. Diabetologia 51:216–226
Sundler F, Hakason R, Lundquist I, Larsson L-I (1977) Effect of alloxan on pancreatic polypeptide (PP) cells. Cell Tissue Res 178:307–312
Rahier J, Wallon J, Loozen A, Lefevre W, Gepts W, Hao J (1983) The pancreatic polypeptide cells in the human pancreas: the effects of age and diabetes. J Clin Endocrinol Metab 56:441–444
Acknowledgments
The authors acknowledge the excellent work of the staff of the Yale PET Center and Tim Mulnix, PhD for the rat holder and technical assistance critical to the success of these studies. These studies were supported by the Yale-Pfizer Bioimaging Alliance and the Juvenile Diabetes Research Foundation (1 37-2009-29). This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Conflicts of Interest/Disclosures
Walter C. Soeller owns shares in Pfizer, Inc. and Judith L. Treadway is an employee and shareholder of Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singhal, T., Ding, YS., Weinzimmer, D. et al. Pancreatic Beta Cell Mass PET Imaging and Quantification with [11C]DTBZ and [18F]FP-(+)-DTBZ in Rodent Models of Diabetes. Mol Imaging Biol 13, 973–984 (2011). https://doi.org/10.1007/s11307-010-0406-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0406-x