Abstract
Introduction
Itaconic acid (ITA) has recently been identified as an antimicrobial metabolite in mammalian immune cells. The presence of ITA was also reported in different tissues of marine molluscs, indicating its role as an endogenous metabolite of molluscs. In addition, the accumulation of ITA has been observed in different tissues of mussels following pathogen challenges. However, the concentration of ITA in mussel tissues and the possible role of this metabolite in the molluscan innate immune system remain unknown.
Objectives
This study aims to quantitatively measure ITA levels in different tissues of marine mussels following an experimental challenge with Vibrio sp. DO1 isolate, and to identify the antimicrobial role of ITA in the innate immune system through the measurement of metabolic and immune alterations in tissues following the challenge.
Methods
In this study, adult Perna canaliculus mussels were experimentally challenged with a pathogenic Vibrio sp. DO1 isolate. The metabolite profiles of five different tissues, including mantle, gill, muscle, hepatopancreas and haemolymph were obtained, and levels of ITA in each tissue were characterized using a gas chromatography–mass spectrometry (GC–MS) metabolomics approach. Flow cytometry was also employed to measure cell health parameters, including oxidative stress via reactive oxygen species (ROS) production, apoptosis via changes in mitochondrial membrane potential (MMP) and haemocyte viability.
Results
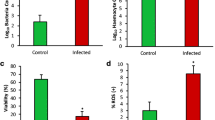
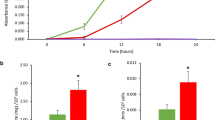
The ITA levels in mantle, gill, muscle and hepatopancreas tissues at 18-h post infection (hpi) with Vibrio sp. were 40.31, 41.71, 11.61 and 41.66 ng mg−1, respectively. In haemolymph, the level of ITA was significantly increased from 95.25 ng ml−1 at 6 hpi to 174.36 ng ml−1 at 18 hpi and 572.12 ng ml−1 at 60 hpi. In line with the accumulation of ITA, we observed increased levels of metabolites within the tricarboxylic acid (TCA) cycle, anti-inflammatory metabolites and alterations of other metabolites associated with immune responses of the host. The flow cytometry analyses revealed increases in ROS production, apoptotic cells and decreases in cell viability.
Conclusions
We reported on the production of ITA in different tissues of P. canaliculus mussels challenged with a marine pathogen which confirmed ITA as an antimicrobial metabolite. The findings revealed insights into the biosynthesis of ITA and suggests its role in antimicrobial and anti-inflammatory activities in the innate immune system. This study also provided insights into the innate immune system of bivalves and highlighted the potential use of ITA as a biomarker for shellfish health assessment in aquaculture.





Similar content being viewed by others
References
Aggio, R., Villas-Boas, S. G., & Ruggiero, K. (2011). Metab: An R package for high-throughput analysis of metabolomics data generated by GC-MS. Bioinformatics, 27, 2316–2318. https://doi.org/10.1093/bioinformatics/btr379.
Alfaro, A. C., Nguyen, T. V., & Mellow, D. (2019). A metabolomics approach to assess the effect of storage conditions on metabolic processes of New Zealand surf clam (Crassula aequilatera). Aquaculture, 498, 315–321. https://doi.org/10.1016/j.aquaculture.2018.08.065.
Allam, B., & Raftos, D. (2015). Immune responses to infectious diseases in bivalves. Journal of Invertebrate Pathology, 131, 121–136. https://doi.org/10.1016/j.jip.2015.05.005.
Bakker, J., Nijsten, M. W. N., & Jansen, T. C. (2013). Clinical use of lactate monitoring in critically ill patients. Annals of Intensive Care, 3, 12–12. https://doi.org/10.1186/2110-5820-3-12.
Bentley, R., & Thiessen, C. P. (1957). Biosynthesis of itaconic acid in Aspergillus terreus III. The properties and reaction mechanism of cis-aconitic acid decarboxylase. Journal of Biological Chemistry, 226, 703–720.
Bonnarme, P., Gillet, B., Sepulchre, A., Role, C., Beloeil, J., & Ducrocq, C. (1995). Itaconate biosynthesis in Aspergillus terreus. Journal of Bacteriology, 177, 3573–3578.
Chong, J., et al. (2018). MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Research, 46, W486–W494. https://doi.org/10.1093/nar/gky310.
Cordes, T., Michelucci, A., & Hiller, K. (2015). Itaconic acid: The surprising role of an industrial compound as a mammalian antimicrobial metabolite. Annual Review of Nutrition, 35, 451–473.
Dull, T. A., & Henneman, P. H. (1963). Urinary hydroxyproline as an index of collagen turnover in bone. New England Journal of Medicine, 268, 132–134.
Etherington, D. J., & Sims, T. J. (1981). Detection and estimation of collagen. Journal of the Science of Food and Agriculture, 32, 539–546.
Gerdol, M., et al. (2018). Immunity in molluscs: Recognition and effector mechanisms, with a focus on bivalvia. In E. Cooper (Ed.), Advances in comparative immunology (pp. 225–341). Cham: Springer.
Goodman, S. (1995). Organic acidemias due to defects in lysine oxidation: 2-ketoadipic acidemia and glutaric acidemia. The metabolic bases of inherited disease. New York: McGraw-Hill.
Grabley, S., & Thiericke, R. (1999). Bioactive agents from natural sources: Trends in discovery and application. Thermal Biosensors, Bioactivity, Bioaffinitty, 64, 101–154.
Grandiosa, R., et al. (2018). Multi-strain probiotics enhance immune responsiveness and alters metabolic profiles in the New Zealand black-footed abalone (Haliotis iris). Fish & Shellfish Immunology, 82, 330–338. https://doi.org/10.1016/j.fsi.2018.08.034.
Hillier, S., & Charnetzky, W. (1981). Glyoxylate bypass enzymes in Yersinia species and multiple forms of isocitrate lyase in Yersinia pestis. Journal of Bacteriology, 145, 452–458.
Kaviarasan, T., Siva, S. R., & Yogamoorthi, A. (2012). Antimicrobial secondary metabolites from marine gastropod egg capsules and egg masses. Asian Pacific Journal of Tropical Biomedicine, 2, 916.
Liu, X., Ji, C., Zhao, J., Wang, Q., Li, F., & Wu, H. (2014). Metabolic profiling of the tissue-specific responses in mussel Mytilus galloprovincialis towards Vibrio harveyi challenge. Fish & Shellfish Immunology, 39, 372–377. https://doi.org/10.1016/j.fsi.2014.05.033.
Liu, X., Zhao, J., Wu, H., & Wang, Q. (2013). Metabolomic analysis revealed the differential responses in two pedigrees of clam Ruditapes philippinarum towards Vibrio harveyi challenge. Fish & Shellfish Immunology, 35, 1969–1975. https://doi.org/10.1016/j.fsi.2013.09.037.
López-Armada, M. J., Riveiro-Naveira, R. R., Vaamonde-García, C., & Valcárcel-Ares, M. N. (2013). Mitochondrial dysfunction and the inflammatory response. Mitochondrion, 13, 106–118.
Lu, J., Shi, Y., Cai, S., & Feng, J. (2017). Metabolic responses of Haliotis diversicolor to Vibrio parahaemolyticus infection. Fish & Shellfish Immunology, 60, 265–274. https://doi.org/10.1016/j.fsi.2016.11.051.
Lu, J., Shi, Y., Wang, S., Chen, H., Cai, S., & Feng, J. (2016). NMR-based metabolomic analysis of Haliotis diversicolor exposed to thermal and hypoxic stresses. Science of the Total Environment, 545–546, 280–288. https://doi.org/10.1016/j.scitotenv.2015.12.071.
McFadden, B., & Purohit, S. (1977). Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. Journal of Bacteriology, 131, 136–144.
Michelucci, A., et al. (2013). Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proceedings of the National Academy of Sciences, 110, 7820–7825.
Mills, E., & O’Neill, L. A. (2014). Succinate: A metabolic signal in inflammation. Trends in Cell Biology, 24, 313–320.
Mills, E. L., et al. (2016). Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell, 167, 457–470. https://doi.org/10.1016/j.cell.2016.08.064.
Mills, E. L., et al. (2018). Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature, 556, 113.
Nguyen, T. V., Alfaro, A. C., Merien, F., Lulijwa, R., & Young, T. (2018a). Copper-induced immunomodulation in mussel (Perna canaliculus) haemocytes. Metallomics, 10, 965–978. https://doi.org/10.1039/c8mt00092a.
Nguyen, T. V., Alfaro, A. C., Merien, F., & Young, T. (2019a). In vitro study of apoptosis in mussel (Perna canaliculus) haemocytes induced by lipopolysaccharide. Aquaculture, 503, 8–15. https://doi.org/10.1016/j.aquaculture.2018.12.086.
Nguyen, T. V., Alfaro, A. C., Merien, F., Young, T., & Grandiosa, R. (2018b). Metabolic and immunological responses of male and female New Zealand Greenshell™ mussels (Perna canaliculus) during Vibrio sp. infection. Journal of Invertebrate Pathology, 157, 80–89. https://doi.org/10.1016/j.jip.2018.08.008.
Nguyen, T. V., Alfaro, A. C., Young, T., Green, S., Zarate, E., & Merien, F. (2019b). Itaconic acid inhibits growth of a pathogenic marine Vibrio strain: A metabolomics approach. Scientific Reports, 9, 5937. https://doi.org/10.1038/s41598-019-42315-6.
Nguyen, T. V., Alfaro, A. C., Young, T., & Merien, F. (2018c). Tissue-specific immune responses to Vibrio sp. infection in mussels (Perna canaliculus): A metabolomics approach. Aquaculture, 500, 118–125. https://doi.org/10.1016/j.aquaculture.2018.09.061.
Nguyen, T. V., Alfaro, A. C., Young, T., Ravi, S., & Merien, F. (2018d). Metabolomics study of immune responses of New Zealand greenshell™ mussels (Perna canaliculus) infected with pathogenic Vibrio sp. Marine Biotechnology, 20, 396–409. https://doi.org/10.1007/s10126-018-9804-x.
Ofulue, A. F., & Thurlbeck, W. M. (1988). Experimental diabetes and the lung: II. In vivo connective tissue metabolism. American Review of Respiratory Disease, 138, 284–289.
Pati, P., Sahu, B. K., & Panigrahy, R. (2015). Marine molluscs as a potential drug cabinet: An overview. Indian Journal of Geo-Marine Science., 44(7), 961–970.
Rogatzki, M. J., Ferguson, B. S., Goodwin, M. L., & Gladden, L. B. (2015). Lactate is always the end product of glycolysis. Frontiers in Neuroscience, 9, 1–7. https://doi.org/10.3389/fnins.2015.00022.
Rőszer, T. (2014). The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell and Tissue Research, 358, 685–695.
Santhi, V., Sivakumar, V., Mukilarasi, M., & Kannagi, A. (2013). Antimicrobial substances of potential biomedical importance from Babylonia zeylanica. Journal of Chemical and Pharmaceutical Research, 5, 108–115.
Shin, J.-H., et al. (2011). 1H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. Journal of Proteome Research, 10, 2238–2247.
Smart, K. F., Aggio, R. B., Van Houtte, J. R., & Villas-Bôas, S. G. (2010). Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography–mass spectrometry. Nature Protocols, 5, 1709.
Strelko, C. L., et al. (2011). Itaconic acid is a mammalian metabolite induced during macrophage activation. Journal of the American Chemical Society, 133, 16386–16389.
Sugimoto, M., et al. (2012). Non-targeted metabolite profiling in activated macrophage secretion. Metabolomics, 8, 624–633.
Weiss, P. H., & Klein, L. (1969). The quantitative relationship of urinary peptide hydroxyproline excretion to collagen degradation. The Journal of Clinical Investigation, 48, 1–10.
Williams, J. O., Roche, T. E., & McFadden, B. A. (1971). Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry, 10, 1384–1390.
Young, T., et al. (2017). Differential expression of novel metabolic and immunological biomarkers in oysters challenged with a virulent strain of OsHV-1. Developmental and Comparative Immunology, 73, 229–245.
Yue, L., & Yao, H. (2016). Mitochondrial dysfunction in inflammatory responses and cellular senescence: Pathogenesis and pharmacological targets for chronic lung diseases. British Journal of Pharmacology, 173, 2305–2318.
Acknowledgements
We would like to thank Aditya Kesarcodi-Watson from Cawthron Institute for providing the bacterial strain, and Kaiaua mussel farms for supplying the experimental mussels. We also thank Leonie Venter for her assistance with sampling, and the laboratory technicians in the School of Science at the Auckland University of Technology and The University of Auckland for their help and support during this project. This project was funded by the New Zealand Ministry of Business, Innovation and Employment (MBIE) (CAWX1707).
Author information
Authors and Affiliations
Contributions
TVN designed and carried out the experiment, sample preparation, data processing and analyses and writing the manuscript. ACA participated in experimental design and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Van Nguyen, T., Alfaro, A.C. Targeted metabolomics to investigate antimicrobial activity of itaconic acid in marine molluscs. Metabolomics 15, 97 (2019). https://doi.org/10.1007/s11306-019-1556-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1556-8