Abstract
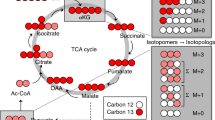
Isotopomer analysis is a very powerful technique for determining site enrichment with stable isotopes. Such information helps determine the relative flux through metabolic pathways. We have developed 1H NMR detection methods to isotopomer analysis of human rhabdomyosarcoma cells grown in the presence of uniformly 13C-labeled glucose. We show that TOCSY can be used both to identify the isotopomer distributions in a substantial number of key compounds and to determine the site-specific enrichment with good precision. Effects of differential relaxation have been specifically addressed. We have identified and quantified isotopomer distributions in Ala, Lactate, (glycolysis markers), nucleotide riboses (pentose phosphate markers), Asp, Glu and Gln (citric acid cycle and anaplerosis markers) as well as in nucleotide pyrimidine rings. Due to the high sensitivity of proton experiments, a reasonable throughput was achieved using a cold probe on only 3–5 mg dry cell weight. This methodology can be applied to biological system using different labeled precursors to examine their metabolic phenotypes and their response to external perturbations.




Similar content being viewed by others
Abbreviations
- PBS:
-
phosphate buffered saline
- TCA:
-
trichloracetic acid
- GC–MS:
-
gas chromatography mass spectrometry
- TOCSY:
-
total correlation spectroscopy
- AXP, GXP, UXP, CXP:
-
mixed adenosine, guanine, cytosine and uridine phosphates (X=M, D or T).
References
Anousis N., Carvalho R.A., Zhao P.Y., Malloy C.R., Sherry A.D. (2004). Compartmentation of glycolysis and glycogenolysis in the perfused rat heart. NMR Biomed. 7, 51–59
Birkemeyer C., Luedemann A., Wagner C., Erban A., Kopka J. (2005). Metabolome analysis: the potential of in vivo labeling with stable isotopes for metabolite profiling. Trends Biotechnol. 23, 28–33
Bollard M.E., Stanley E.G., Lindon J.C., Nicholson J.K., Holmes E. (2005). NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 18, 143–162
Burgess S.C., Carvalho R.A., Merritt M.E., et al. (2001). C-13 isotopomer analysis of glutamate by J-resolved heteronuclear single quantum coherence spectroscopy. Anal. Biochem. 289, 187–195
Carvalho R.A., Jeffrey F.M.H., Sherry A.D., Malloy C.R. (1998). C-13 isotopomer analysis of glutamate by heteronuclear multiple quantum coherence total correlation spectroscopy (HMQC-TOCSY). FEBS Lett. 440, 382–386
Carvalho R.A., Zhao P., Wiegers C.B., et al. (2001). TCA cycle kinetics in the rat heart by analysis of C-13 isotopomers using indirect H-1 C-13 detection. Am. J. Physiol. Heart Circ. Physiol. 281, H1413–H1421
Chatham J.C., Bouchard B., Des Rosiers C. (2003). A comparison between NMR and GCMS C-13-isotopomer analysis in cardiac metabolism. Mol. Cell. Biochem. 249, 105–112
Cline G.W., LePine R.L., Papas K.K., Kibbey R.G., Shulman G.I. (2004). C-13 NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J. Biol. Chem. 279, 44370–44375
Des Rosiers C., Lloyd S., Comte B., Chatham J.C. (2004). A critical perspective of the use of C-13-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab. Eng. 6, 44–58
Fan T.W.-M., Bandura L.L., Lane A.N., Higashi R.M. (2005). Metabolomics-edited transcriptomics analysis of se anticancer action in human lung cancer cells. Metabolomics 1, 325–339
Fan T.W.M., Colmer T.D., Lane A.N., Higashi R.M. (1993). Determination of metabolites by H-1-NMR and Gc—analysis for organic osmolytes in crude tissue-extracts. Anal. Biochem. 214, 260–271
Fan T.W.M., Higashi R.M., Lane A.N., Jardetzky O. (1986). Combined use of H-1-NMR and GC–MS for metabolite monitoring and in vivo H-1-NMR assignments. Biochim. Biophys. Acta 882, 154–167
Fan T.W.M., Lane A.N., Higashi R.M. (2004). The promise of metabolomics in cancer molecular therapeutics. Curr. Opin. Mol. Ther. 6, 584–592
Foxall P.J.D., Parkinson J.A., Sadler I.H., Lindon J.C., Nicholson J.K. (1993). Analysis of biological-fluids using 600 MHz proton NMR-spectroscopy—application of homonuclear 2-dimensional J-resolved spectroscopy to urine and blood-plasma for spectral simplification and assignment. J. Pharm. Biomed. Anal. 11, 21–31
Griffin J.L., O’Donnell J.M., White L.T., Hajjar R.J., Lewandowski E.D. (2000). Postnatal expression and activity of the mitochondrial 2-oxoglutarate-malate carrier in intact hearts. Am. J. Physiol. Cell Physiol. 279, C1704–C1709
Heijne W.H.M., Lamers R., van Bladeren P.J., et al. (2005). Profiles of metabolites and gene expression in rats with chemically induced hepatic necrosis. Toxicol. Pathol. 33, 425–433
Henry P.G., Oz G., Provencher S., Gruetter R. (2003a). Toward dynamic isotopomer analysis in the rat brain in vivo: automatic quantitation of C-13 NMR spectra using LC model. NMR Biomed. 16, 400–412
Henry P.G., Tkac I., Gruetter R. (2003b). H-1-localized broadband C-13 NMR spectroscopy of the rat brain in vivo at 9.4 T. Magn. Reson. Med. 50, 684–692
Katz-Brull R., Seger D., Rivenson-Segal D., Rushkin E., Degani H. (2002). Metabolic markers of breast cancer: enhanced choline metabolism and reduced choline-ether-phospholipid synthesis. Can. Res. 62, 1966–1970
Khairallah M., Labarthe F., Bouchard B., et al. (2004). Profiling substrate fluxes in the isolated working mouse heart using C-13-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am. J. Physiol. Heart Circ. Physiol. 286, H1461–H1470
Lean C.L., Bourne R., Thompson J.F., et al. (2003). Rapid detection of metastatic melanoma in lymph nodes using proton magnetic resonance spectroscopy of fine needle aspiration biopsy specimens. Melanoma Res. 13, 259–261
Lewandowski E.D., Johnston D.L., Roberts R. (1991). Effects of inosine on glycolysis and contracture during myocardial-ischemia. Circ. Res. 68, 578–587
Lindon J.C., Holmes E., Nicholson J.K. (2004). Metabonomics: systems biology in pharmaceutical research and development. Curr. Opin. Mol. Ther. 6, 265–272
Lloyd S.G., Zeng H.D., Wang P.P., Chatham J.C. (2004). Lactate isotopomer analysis by H-1 NMR spectroscopy: consideration of long-range nuclear spin-spin interactions. Magn. Reson. Med. 51, 1279–1282
London R.E., Allen D.L., Gabel S.A., DeRose E.F. (1999). Carbon-13 nuclear magnetic resonance study of metabolism of propionate by Escherichia coli. J. Bacteriol. 181, 3562–3570
Lu D.H., Mulder H., Zhao P.Y., et al. (2002). C-13 NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc. Natl. Acad. Sci. U.S.A. 99, 2708–2713
Marin S., Lee W.N.P., Bassilian S., et al. (2004). Dynamic profiling of the glucose metabolic network in fasted rat hepatocytes using 1,2-C-13(2) glucose. Biochem. J. 381, 287–294
Puccetti C., Aureli T., Manetti C., Conti F. (2002). C-13-NMR isotopomer distribution analysis: a method for measuring metabolic fluxes in condensation biosynthesis. NMR Biomed. 15, 404–415
Vanzijl P.C.M., Chesnick A.S., Despres D., et al. (1993). In-vivo proton spectroscopy and spectroscopic imaging of (1-C-13)-glucose and its metabolic products. Magn. Reson. Med. 30, 544–551
Vizan P., Boros L.G., Figueras A., et al. (2005). K-ras codon-specific mutations produce distinctive metabolic phenotypes in human fibroblasts. Can. Res. 65, 5512–5515
Zwingmann C., Chatauret N., Leibfritz D., Butterworth R.F. (2003). Selective increase of brain lactate synthesis in experimental acute liver failure: results of a H-1–C-13 nuclear magnetic resonance study. Hepatology 37, 420–428
Zwingmann C., Richter-Landsberg C., Leibfritz D. (2001). C-13 isotopomer analysis of glucose and alanine metabolism reveals cytosolic pyruvate compartmentation as part of energy metabolism in astrocytes. Glia 34, 200–212
Acknowledgments
We thank Laura Bandura for assistance with cell growth. This work was supported by the Kentucky Challenge for Excellence (to ANL). NMR spectra were recorded at the JG Brown Cancer Center NMR Suite supported by the Brown Foundation and NSF EPSCoR grant EPS-0132295 for NMR instrumentation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material
Rights and permissions
About this article
Cite this article
Lane, A.N., Fan, T.W.M. Quantification and identification of isotopomer distributions of metabolites in crude cell extracts using 1H TOCSY. Metabolomics 3, 79–86 (2007). https://doi.org/10.1007/s11306-006-0047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-006-0047-x