Abstract
Kidney transplantation is the preferred treatment for individuals with kidney failure offering improved quality and quantity of life. Despite significant advancements in short term graft survival, longer term survival rates have not improved greatly mediated in large by chronic antibody mediated rejection. Strategies to reduce the donor kidney antigenic load may translate to improved transplant survival. CD39 on the vascular endothelium and on circulating cells, in particular regulatory T cells (Treg), is upregulated in response to hypoxic stimuli and plays a critical role in regulating the immune response removing proinflammatory ATP and generating anti-inflammatory adenosine. Herein, the role of CD39 in reducing ischaemia–reperfusion injury (IRI) and on Treg within the context of kidney transplantation is reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
‘I think scientists are creative people exactly like artists. I am going to treat you like an artist. I want to let you express your creative spirit in whatever way suits you best.’ [extract from 1].
I was humbled and honoured to receive the 2021 Burnstock Oration in recognition of outstanding contributions to purinergic signalling. I had the privilege of speaking with Prof Burnstock during one of his stays in Melbourne — his vitality, vivaciousness, interest, congeniality and superb intellect are vivid memories of this meeting. Vale Professor Geoffrey Burnstock.
I dedicate this award to my mentors and sponsors Professors Tony d’Apice, Simon Robson and Terry Strom who have guided me on my journey in purinergic signalling in kidney transplantation. Tony supervised my PhD studies investing time in me, a novice in CD39 biology and science in general. I am grateful for the wisdom he bestowed, his confidence in my strengths and abilities and encouragement to pursue an academic career. I completed my post doc studies with Simon and Terry, who opened my eyes to wonders of international research, facilitated creative thinking and epitomised leadership. Simon remains a constant source of advice and perspective. I am indebted to Professor Silvia Deaglio for her comradery, collegiality, agile intellect, scientific analysis, collaboration, and friendship during our post doc studies in Boston.
“A mentor is not someone who walks ahead of us and tells us how they did it.
A mentor is someone who walks alongside us to guide us on what we can do.” Simon Sinek.
CD39: the master switch
The dual function of adenosine triphosphate (ATP) as a signalling molecule was first documented in 1962 by Prof Burnstock himself and proclaimed a decade later, almost 50 years after the initial characterisation of ATP as the energy currency of all living cells [reviewed in 2]. Whilst existing in the intracellular space at high concentration, the negative charge of ATP restricts movement across the cell membrane and thus it is only found in micromolar concentrations outside the cells under physiological conditions. In pathological states however, ATP may be extruded from necrotic cells or released during apoptosis in a regulated manner via pannexin hemichannels [3]. Furthermore, endothelial cells and activated inflammatory cells can also release ATP through connexin hemichannels [4]. With the identification of an array of P2X and P2Y receptors and their distribution throughout the body, it has become clear that the biological effects of ATP are diverse and pervasive. In the context of kidney transplantation, ATP in the most part promotes an inflammatory and thrombotic response through a direct action on the kidney endothelium and systemically via activation of inflammatory cells and platelets [4].
The pathophysiological effects of ATP are tightly regulated by the family of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) such as CD39, which rapidly hydrolyse ATP. ATP is hydrolysed to adenosine diphosphate (ADP), which is in turn hydrolysed to adenosine monophosphate (AMP). AMP is degraded to adenosine by CD73. Extracellular adenosine signals through four G-protein coupled P1 receptors which in general oppose the actions of ATP. Furthermore, adenosine via equilibrative nucleoside transporter (ENT) 1 and 2 can move back into the intracellular space, where it is degraded to inosine (via adenosine deaminase) or re-phosphorylated to AMP (via adenosine kinase) [4].
CD39 (also known as NTPDase 1) is the prototype of the four membrane — bound NTPDases and hydrolyses ATP and ADP with equal potency. CD39L1 (also known as NTPDase 2) preferentially hydrolyses ADP over ATP. NTPDase 3 and NTPDase 8 preferentially hydrolyse ATP over ADP [5]. Whilst the CD39-adenosine axis is the predominant purinergic pathway in renal pathophysiology and immune regulation, there are several other enzymes that impact ATP regulation which has been discussed recently [6] and is beyond the focus of this review.
CD39 is expressed widely within the immune system and on the vascular endothelium. In the kidney, CD39 is expressed on vascular endothelium and within the collecting ducts in both the cortex and the medulla. It is also expressed in the ascending thin limb of the loop of Henle [7, 8] and the glomerulus [8]. CD73 is predominantly expressed in the glomeruli, on the endothelium and peritubular fibroblasts [9]. Within the kidney, the enzymatic efficiency of CD73 is less than that of CD39 [10] resulting in ATP being degraded more rapidly than adenosine is generated, which highlights the importance of both the removal of nucleotides and production of adenosine in kidney disease.
Adenosine is the canonical ligand of the four G-protein-coupled P1 receptors known as the A1, A2A, A2B and A3 receptors. Adenosine receptors are classified based on their differential coupling to adenylyl cyclase to regulate cyclic adenosine monophosphate (cAMP) levels: A1 and A3 receptors are coupled to the G-inhibitory subunit and the activation leads to a reduction in the level of intracellular cAMP, whereas as A2A and A2B receptors are coupled to the G-stimulatory subunit and their activation results in an increase in intracellular cAMP. Signalling through these receptors is in fact far more complex and adenosine receptors can also modulate a variety of additional second messengers. For example, the A2B receptor also couples to Gq proteins, which stimulate phospholipase C activity and intracellular calcium mobilisation [11, 12]. The A2B receptor has lower affinity and thus high pericellular concentrations of adenosine, as seen in pathological states, are required compared to the other P1 receptors [12]. Furthermore, the A2B receptor has also been shown to interact with AMP via computational modelling, in vitro assays [13] and in vivo studies [14]. An additional layer of complexity exists with receptor dimerization which may be necessary for full receptor functionality. For example, heterodimerization of the A2A and A2B receptors is necessary for the A2B receptor to be efficiently expressed on the cell surface in vitro [15].
The ATP — CD39 — adenosine axis functions as a dynamic pathway [16] that regulates the availability of ATP and adenosine for P2 and P1 receptor activation respectively in the extracellular milieu. CD39 thus has profound implications in kidney transplantation outcomes, in the most part through modifying the antigenicity of the donor kidney and thus the recipient immune response. Antibody mediated rejection is the leading cause of kidney failure following transplantation [17]. Whilst many factors that increase the risk of antibody mediated rejection are non-modifiable, such as donor and recipient age [18], others such as ischaemia reperfusion injury (IRI) [19] present an opportunity for manipulation. Furthermore, regulatory T cells (Treg) are central to preventing kidney transplant rejection and, in animal models, are a potential therapeutic target [20]. This review will focus on the CD39 — adenosine axis in models of kidney IRI and on Treg.
Ischaemia reperfusion injury (IRI)
IRI is an obligatory insult in the kidney transplantation process occurring with organ procurement and engraftment. Warm ischaemia occurs in the context of organ procurement; cold ischaemia uniquely occurs in transplantation where the donor organ undergoes cold preservation, which can be prolonged for many hours. The re-establishment of blood flow at the time of engraftment paradoxically incites further inflammation despite terminating hypoxic injury. The clinical implications of IRI include systemic inflammatory effects, organ dysfunction encompassing delayed graft function, acute allograft rejection and chronic allograft nephropathy.
Purinergic signalling has been implicated in both the pathogenesis of, and the immune response to, IRI [21]. Hypoxic injury causes a dramatic rise in P2X7 receptor expression [22] and the pericellular concentration of ATP (released from injured or dying cells), exaggerated by the loss of CD39 and CD73 from activated endothelial cells [23], which ignites an inflammatory response. Indeed, mice deficient in Cd39 are highly susceptible to kidney injury [24]. Conversely, mice that over-express human CD39 [25] are robustly protected in models of both warm [26] and cold ischaemia [27] an effect that persists whether CD39 over-expression is restricted to the vascular endothelium or the circulating cells. CD39 activity can be augmented pharmacologically with soluble CD39 [26, 28] given prior to ischaemia which also mitigated injury. Such an effect can be simulated through the process of ischaemic preconditioning whereby repeated short episodes of ischaemia (4 min) are followed by short periods of reperfusion (4 min) prior to extended ischaemia and reperfusion [29]. Ischaemic preconditioning resulted in an increase in CD39 transcript expression and pericellular adenosine concentration [29] mediated via specificity protein 1 (Sp1) [30], a ubiquitously expressed transcription factor implicated in promoting hypoxic gene transcription. Intriguingly protection from IRI is conferred with inhaled carbon monoxide, an effect that is dependent on the induction of CD39 expression, within 2 h of reperfusion and which is ineffective in mice deficient in Cd39 [31]. This effect was mediated through upregulation and stabilisation of circadian rhythm protein Period 2 (Per2) and serum erythropoietin (EPO) [31].
Whilst ATP promoted kidney IRI predominantly via the P2X7 receptor [22], an effect which can be abrogated by administration of a P2X7 receptor antagonist up to 6 h post reperfusion [32], CD39-adenosine protection is mediated via A1, A2A and A2B receptors depending on the predominant cell type involved [reviewed in 33]. A1 receptor activation on proximal tubular cells ameliorated ischemic-induced acute kidney injury with reduced apoptosis, necrosis and inflammation [34, 35]. Stimulation of the A2A receptor on circulating CD4+ T cells mediated protection from IRI, an effect that was IFNγ dependent [36]. Additional studies have shown that A2A receptor activation on Treg and [37] and dendritic cells [38] protected against kidney IRI. Treg play a crucial role in IRI — depletion of Treg worsened IRI whereas the infusion of Treg enhanced the repair response [reviewed in 39]. On the vascular endothelium, the A2B receptor was upregulated within 24 h [31, 40] and played a predominant role in mediating protection against kidney IRI [41].
Whilst much of the impact of CD39 has been attributed to the removal of ATP and generation of adenosine, AMP is a necessary intermediary. Our work has shown that AMP concentrations increased immediately following kidney ischaemia [26] and mice treated with a CD73 inhibitor or deficient in CD73 are paradoxically protected in a model of mild kidney IRI [42], an effect mediated by the A2B receptor [14].
In addition to increasing the immunogenicity of the transplanted kidney, IRI portends to chronic kidney disease which impacts graft function. Purinergic signalling again plays a significant role in chronic kidney disease which is characterised histologically by kidney fibrosis. The fibroblast expresses CD73 and A2B receptor [43] and agonism of A2B receptor increased transcription of profibrotic and inflammatory mediators [44]. Indeed, CD73 and A2B receptor [45] and IL-6 [46] were significantly elevated in the kidneys of individuals with chronic kidney disease and further elevated in those with hypertension. So, whilst a transient increase in CD39 protects acutely and prevents chronic kidney disease, the sustained generation of adenosine through the concerted and persistent action of CD39 and CD73 leads to increased myofibroblast activity and fibrosis [26, 47].
Regulatory T cells (Treg)
Whilst several studies have shown a positive correlation between the presence of Treg and clinical outcomes in kidney transplantation [48, 49], the function, phenotype and biology of Treg continue to evolve [reviewed in 50]. Initially described by using solely the intensity of CD25 expression to identify CD4+CD25hi Treg, a significant advancement came with the identification of the transcription factor FOXP3 necessary for Treg survival and suppressive function. Further differentiation markers have been identified including CD39 [51, 52], which is of functional significance facilitating adenosine generation and, via A2A receptor, halting cellular proliferation [52, 53]. Indeed, Treg from mice lacking Cd39 have suboptimal suppressive capacity and resulted in more rapid rejection of allogenic skin grafts [52] and the spontaneous development of autoimmune diseases associated with Th1 immune deviation [54].
In humans, four T cell populations can be defined by the differential expression of CD4, CD25 and CD39. Notably, unlike in mice, human Treg cells do not express CD73, implicating a paracrine mechanism for the generation of adenosine whereby CD73 is expressed on target cells [55]. CD39 expression on Treg is increased by a positive feedback loop, in which the Cd39 promoter is transactivated by adenosine [56]. CD39 expression also decreases Treg cell susceptibility to ATP-induced cell death [57]. The presence of CD39 denoted memory cells (effector (mTeff) and regulatory (mTreg) in 1:1) [55] whereas the absence of CD39 on CD4+CD25+ cells predicted cells with Th17 potential [55, 58]. Using these markers, T cell number and dynamics can be tracked before and after transplantation and in the setting of stable graft function remain static [55]. However, the kinetics of Treg differ and in the presence of stable graft function, the immunosuppressive capacity of CD4+CD25+CD39+ Treg was enhanced compared to healthy controls [59]. This was even more apparent in individuals deemed tolerant. Here, memory Treg were increased, had greater suppressive function and CD39 expression and an enhanced capacity to degrade extracellular ATP compared to those with stable function [60, 61]. These data contrast with the situation of antibody-mediated rejection characterised by inflammation in which the effector: memory T cell ratio is heavily skewed to effector cells [55].
Since the original description of CD39 on Treg [51, 52], CD39 has been identified on T regulatory type 1 cells (Tr1) cells — a subset of Treg characterised by the secretion of IL-10 and the lack of FOXP3 expression. CD39 was essential for the full suppressive activity of Tr1 cells in vitro and in vivo [62] and in human kidney transplantation, the numbers of Tr1 cells were positively correlated with stable graft function [63]. Moreover, CD39 promoted Tr1 cell differentiation by limiting extracellular ATP [62]. Another T cell subset with suppressive capabilities includes suppressive Th17 cells that have high levels of CD39, co-express CD73 and generate adenosine [64]. These CD39 expressing subsets have non-redundant roles in experimental mouse model of autoimmune encephalomyelitis [62] and in humans with Crohn’s disease [64]. A role for suppressive Th17 cells in kidney transplantation however is yet to be defined.
Conclusion
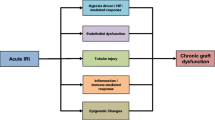
Kidney transplantation is the preferred treatment for individuals with kidney failure offering improved quality and quantity of life. Despite significant advancements in short term graft survival, longer term survival rates have not improved greatly mediated in large by chronic antibody mediated rejection. Strategies to reduce the donor kidney antigenic load may translate to improved transplant survival. CD39 on the vascular endothelium and on circulating cells, in particular Treg, is upregulated in response to hypoxic stimuli and plays a critical role in regulating the immune response removing proinflammatory ATP and generating anti-inflammatory adenosine (Fig. 1). Augmenting CD39 in the peri-transplant period can mitigate IRI and high expression of CD39 on mTreg may promote kidney transplant tolerance.
CD39-rich milieu improves outcomes in kidney transplantation. In IRI, ATP is released from inflammatory and apoptotic cells via connexin and pannexin hemi-channels (blue rectangle) or directly from necrotic cells into the extracellular space. ATP is converted through an enzymatic process by CD39 and CD73 on the endothelium to adenosine (ADO). Adenosine mediates its anti-inflammatory effects via A2A receptor (A2AR) on T regulatory cells (Treg) and T effector cells (Teff) and A2B receptor (A2BR) on the endothelium. CD39 activity may be increased by ischaemic preconditioning, over-expression, carbon monoxide or delivery of soluble CD39. Improved kidney transplant outcomes occur with greater circulating numbers of Tr1 and memory Treg (mTreg), both of which express CD39
Data availability
Upon request.
References
G. Burnstock, “Professor Geoffrey Burnstock, neurobiologist,” in Conversations with Australian Scientists, R. Williams, Ed., ed. Canberra: Australian Academy of Science, 2008.
B. S. Khakh and G. Burnstock, “The double life of ATP,” Sci Am, vol. 301, no. 6, pp. 84-90, 92, Dec 2009, https://doi.org/10.1038/scientificamerican1209-84.
Lohman AW, Billaud M, Isakson BE (2012) Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res 95(3):269–280. https://doi.org/10.1093/cvr/cvs187
Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during inflammation. N Engl J Med 367(24):2322–2333. https://doi.org/10.1056/NEJMra1205750
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2(2):409–430. https://doi.org/10.1007/s11302-006-9003-5
Dwyer KM, Kishore BK, Robson SC (2020) Conversion of extracellular ATP into adenosine: a master switch in renal health and disease. Nat Rev Nephrol 16(9):509–524. https://doi.org/10.1038/s41581-020-0304-7
Kishore BK et al (2005) Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288(5):F1032–F1043. https://doi.org/10.1152/ajprenal.00108.2004
Vekaria RM, Shirley DG, Sevigny J, Unwin RJ (2006) Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol 290(2):F550–F560. https://doi.org/10.1152/ajprenal.00151.2005
Le Hir M, Kaissling B (1993) Distribution and regulation of renal ecto-5’-nucleotidase: implications for physiological function of adenosine. Am J Physiol 264(3 Pt 2):F377–F387
J. Karczewska, L. Martyniec, G. Dzierzko, J. Stepinski, and S. Angielski, “The relationship between constitutive ATP release and its extracellular metabolism in isolated rat kidney glomeruli,” J Physiol Pharmacol, vol. 58, no. 2, pp. 321–33, Jun 2007. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/17622700.
Blackburn MR, Vance CO, Morschl E, Wilson CN (2009) Adenosine receptors and inflammation. Handb Exp Pharmacol 193:215–269. https://doi.org/10.1007/978-3-540-89615-9_8
B. B. Fredholm, I. J. AP, K. A. Jacobson, K. N. Klotz, and J. Linden, “International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors,” Pharmacol Rev, vol. 53, no. 4, pp. 527–52, Dec 2001. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/11734617.
Holien JK et al (2018) AMP and adenosine are both ligands for adenosine 2B receptor signaling. Bioorg Med Chem Lett 28(2):202–206. https://doi.org/10.1016/j.bmcl.2017.11.019
S. Rajakumar, B. Lu, S. Crikis, A. d'Apice, P. J. Cowan, and K. M. Dwyer, “CD73-deficiency protects in kidney ischemia reperfusion injury (IRI) - the role of adenosine, A1, A2A and A2B receptors,” Nephrology (Carlton), p. 49, 2011.
Moriyama K, Sitkovsky MV (2010) Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem 285(50):39271–39288. https://doi.org/10.1074/jbc.M109.098293
Kishore BK, Robson SC, Dwyer KM (2018) CD39-adenosinergic axis in renal pathophysiology and therapeutics. Purinergic Signal 14(2):109–120. https://doi.org/10.1007/s11302-017-9596-x
Sellares J et al (2012) Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12(2):388–399. https://doi.org/10.1111/j.1600-6143.2011.03840.x
Dorje C et al (2013) Early versus late acute antibody-mediated rejection in renal transplant recipients. Transplantation 96(1):79–84. https://doi.org/10.1097/TP.0b013e31829434d4
Goto R, Issa F, Heidt S, Taggart D, Wood KJ (2013) Ischemia-reperfusion injury accelerates human antibody-mediated transplant vasculopathy. Transplantation 96(2):139–145. https://doi.org/10.1097/TP.0b013e318295ee32
Hu M et al (2013) Infiltrating Foxp3(+) regulatory T cells from spontaneously tolerant kidney allografts demonstrate donor-specific tolerance. Am J Transplant 13(11):2819–2830. https://doi.org/10.1111/ajt.12445
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion–from mechanism to translation. Nat Med 17(11):1391–1401. https://doi.org/10.1038/nm.2507
Qian Y et al (2021) P2X7 receptor signaling promotes inflammation in renal parenchymal cells suffering from ischemia-reperfusion injury. Cell Death Dis 12(1):132. https://doi.org/10.1038/s41419-020-03384-y
Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K (2005) Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost 31(2):217–233. https://doi.org/10.1055/s-2005-869527
Lu B et al (2008) The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation 86(12):1707–1712. https://doi.org/10.1097/TP.0b013e31819022bc
Dwyer KM et al (2004) Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest 113(10):1440–1446. https://doi.org/10.1172/JCI19560
Roberts V, Campbell DJ, Lu B, Chia J, Cowan PJ, Dwyer KM (2017) The differential effect of apyrase treatment and hCD39 overexpression on chronic renal fibrosis after ischemia-reperfusion injury. Transplantation 101(7):e194–e204. https://doi.org/10.1097/TP.0000000000001679
Crikis S et al (2010) Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant 10(12):2586–2595. https://doi.org/10.1111/j.1600-6143.2010.03257.x
Sashindranath M et al (2017) Development of a novel strategy to target CD39 antithrombotic activity to the endothelial-platelet microenvironment in kidney ischemia-reperfusion injury. Purinergic Signal 13(2):259–265. https://doi.org/10.1007/s11302-017-9558-3
Grenz A et al (2007) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21(11):2863–2873. https://doi.org/10.1096/fj.06-7947com
Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP (2009) Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113(1):224–232. https://doi.org/10.1182/blood-2008-06-165746
Correa-Costa M et al (2018) Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci U S A 115(10):E2302–E2310. https://doi.org/10.1073/pnas.1716747115
Yan Y et al (2015) P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol Cell Physiol 308(6):C463–C472. https://doi.org/10.1152/ajpcell.00245.2014
Roberts V, Lu B, Rajakumar S, Cowan PJ, Dwyer KM (2013) The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal 9(2):135–143. https://doi.org/10.1007/s11302-012-9342-3
Lee HT, Emala CW (2000) Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am J Physiol Renal Physiol 278(3):F380–F387. https://doi.org/10.1152/ajprenal.2000.278.3.F380
Lee HT, Gallos G, Nasr SH, Emala CW (2004) A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15(1):102–111. https://doi.org/10.1097/01.asn.0000102474.68613.ae
Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD (2006) Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol 176(5):3108–3114. https://doi.org/10.4049/jimmunol.176.5.3108
Kinsey GR et al (2012) Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol 23(9):1528–1537. https://doi.org/10.1681/ASN.2012010070
Li L et al (2012) Dendritic cells tolerized with adenosine A(2)AR agonist attenuate acute kidney injury. J Clin Invest 122(11):3931–3942. https://doi.org/10.1172/JCI63170
GharaieFathabad S et al (2020) T lymphocytes in acute kidney injury and repair. Semin Nephrol 40(2):114–125. https://doi.org/10.1016/j.semnephrol.2020.01.003
Roberts V, Lu B, Dwyer KM, Cowan PJ (2014) Adenosine receptor expression in the development of renal fibrosis following ischemic injury. Transplant Proc 46(10):3257–3261. https://doi.org/10.1016/j.transproceed.2014.09.151
Grenz A et al (2008) The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5(6):e137. https://doi.org/10.1371/journal.pmed.0050137
Rajakumar SV et al (2010) Deficiency or inhibition of CD73 protects in mild kidney ischemia-reperfusion injury. Transplantation 90(12):1260–1264. https://doi.org/10.1097/TP.0b013e3182003d9b
Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y (2011) A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol 22(5):890–901. https://doi.org/10.1681/ASN.2010080890
P. F. Wilkinson, F. X. Farrell, D. Morel, W. Law, and S. Murphy, “Adenosine signaling increases proinflammatory and profibrotic mediators through activation of a functional adenosine 2B receptor in renal fibroblasts,” Ann Clin Lab Sci, vol. 46, no. 4, pp. 339–45, Jul 2016. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/27466291.
Zhang W et al (2013) Elevated ecto-5’-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112(11):1466–1478. https://doi.org/10.1161/CIRCRESAHA.111.300166
Zhang W et al (2012) Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 59(1):136–144. https://doi.org/10.1161/HYPERTENSIONAHA.111.173328
Roberts VS, Cowan PJ, Alexander SI, Robson SC, Dwyer KM (2014) The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int 86(4):685–692. https://doi.org/10.1038/ki.2014.244
Bestard O et al (2008) Presence of FoxP3+ regulatory T cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol 19(10):2020–2026. https://doi.org/10.1681/ASN.2007111174
Bestard O et al (2011) Intragraft regulatory T cells in protocol biopsies retain foxp3 demethylation and are protective biomarkers for kidney graft outcome. Am J Transplant 11(10):2162–2172. https://doi.org/10.1111/j.1600-6143.2011.03633.x
Braza F, Durand M, Degauque N, Brouard S (2015) Regulatory T cells in kidney transplantation: new directions? Am J Transplant 15(9):2288–2300. https://doi.org/10.1111/ajt.13395
Borsellino G et al (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110(4):1225–1232. https://doi.org/10.1182/blood-2006-12-064527
Deaglio S et al (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204(6):1257–1265. https://doi.org/10.1084/jem.20062512
Hasko G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7(9):759–770. https://doi.org/10.1038/nrd2638
Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC (2007) CD39 and control of cellular immune responses. Purinergic Signal 3(1–2):171–180. https://doi.org/10.1007/s11302-006-9050-y
Dwyer KM et al (2010) Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant 10(11):2410–2420. https://doi.org/10.1111/j.1600-6143.2010.03291.x
Liao H, Hyman MC, Baek AE, Fukase K, Pinsky DJ (2010) cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. J Biol Chem 285(19):14791–14805. https://doi.org/10.1074/jbc.M110.116905
Aswad F, Kawamura H, Dennert G (2005) High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol 175(5):3075–3083. https://doi.org/10.4049/jimmunol.175.5.3075
Zhou Q et al (2009) Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant 9(10):2303–2311. https://doi.org/10.1111/j.1600-6143.2009.02777.x
McRae JL, Chia JS, Pommey SA, Dwyer KM (2017) Evaluation of CD4(+) CD25(+/-) CD39(+) T-cell populations in peripheral blood of patients following kidney transplantation and during acute allograft rejection. Nephrology (Carlton) 22(7):505–512. https://doi.org/10.1111/nep.12894
Durand M et al (2018) Increased degradation of ATP is driven by memory regulatory T cells in kidney transplantation tolerance. Kidney Int 93(5):1154–1164. https://doi.org/10.1016/j.kint.2017.12.004
Braza F et al (2015) Central role of CD45RA- Foxp3hi memory regulatory T cells in clinical kidney transplantation tolerance. J Am Soc Nephrol 26(8):1795–1805. https://doi.org/10.1681/ASN.2014050480
Mascanfroni ID et al (2015) Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med 21(6):638–646. https://doi.org/10.1038/nm.3868
Song Y, Wang N, Chen L, Fang L (2021) Tr1 cells as a key regulator for maintaining immune homeostasis in transplantation. Front Immunol 12:671579. https://doi.org/10.3389/fimmu.2021.671579
Longhi MS et al (2014) Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS ONE 9(2):e87956. https://doi.org/10.1371/journal.pone.0087956
Acknowledgements
I am privileged to have had the opportunity to supervise several HDR students all of whom have gone on to successful independent careers.
“Every beginner possesses a great potential to be an expert in his or her chosen field.”
― Lailah Gifty Akita, Think Great: Be Great!
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
N/A.
Informed consent
N/A.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dwyer, K.M. Burnstock oration — purinergic signalling in kidney transplantation. Purinergic Signalling 18, 387–393 (2022). https://doi.org/10.1007/s11302-022-09865-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09865-3