Abstract
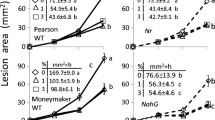
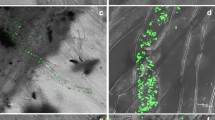
In order to define the role of oxalic acid (OA) in the invasion of Botrytis cinerea in tomato plants, the OA induction of resistance related to oxalate oxidase (O×O) and germin was examined. In greenhouse experiments, OA at 3 mmol/L significantly induced resistance in tomato plants against B. cinerea strains B05.10 and T4, reducing lesion size of 37.55% and 24.91% by compared with distilled water control, respectively, while 20 mmol/L OA increasing by 36.14% and 41.48%. OA contents were 98 and 46 µg/mL when tomato plants were infected by B. cinerea strains B05.10 and T4, respectively. To define the molecular-genetic mechanisms, we compared the gene expression under four different conditions: 3 mmol/L OA-treated plants, 20 mmol/L OA-treated plants, B. cinerea strain B05.10-infected plants (B05.10 Inf plants) and B. cinerea strain T4-infected plants (T4 Inf plants). In 3 mmol/L OA-treated plants, the expressions of O×O and Germin peaked at 48 h after spraying, with approximate threefold and 18-fold increase compared with the control expression, respectively. In T4 Inf plants, the expression (mRNA accumulation) of O×O and Germin reached the highest levels at 24 h after inoculation, with 3- and 13-times that immediately after inoculation, respectively. In total, these findings suggest that elevated levels of OA correlated with increased fungal invasion and lower OA induced resistance in tomato plants by increasing expressions of O×O and Germin.



Similar content being viewed by others
References
Bateman DF, Beer SV (1965) Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotium rolfsii. Phytopathology 55:204–211
Beracochea VC, Almasia NI, Peluffo L, Nahirnak V, Hoop EH, Paniego N, Heinz RA, Vazquez-Rovere C, Lia VV (2015) Sunflower germin-like protein HaGLP1 promotes ROS accumulation and enhances protection against fungal pathogens in transgenic Arabidopsis thaliana. Plant Cell Rep 34:1717–1733
Berna A, Bernier F (1999) Regulation by biotic and abiotic stress of a wheat germin encoding oxalate oxidase, a H2O2-producing enzyme. Plant Mol Biol 39:539–549
Caliskan M, Cuming AC (1998) Spatial specificity of H2O2-generating oxalate oxidase gene expression during wheat embryo germination. Plant J 15:165–171
Cessna SG, Sears VE, Dickman MB, Low PS (2000) Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 12:2191–2199
Chalupowicz L, Cohen-Kandli M, Dror O, Eichenlaub R, Gartemann KH, Sessa G, Barash I, Manulis-Sasson S (2010) Sequential expression of bacterial virulence and plant defense genes during infection of tomato with Clavibacter michiganensis subsp. michiganensis. Phytopathology 100:252–261
Dahiya T, Yadav S, Chauhan N, Handa P, Pundir CS (2010) Strawberry fruit oxalate oxidase-detection, purification, characterization and physiological role. J Plant Biochem Biotechnol 19:247–250
Davidson AL, Blahut-Beatty L, Itaya A, Zhang Y, Zheng S, Simmonds D (2016) Histopathology of Sclerotinia sclerotiorum infection and oxalic acid function in susceptible and resistant soybean. Plant Pathol 65:878–887
Daymi C, Angel GC, Alexander M (2016) Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol Biochem 103:10–23
Derckel JP, Baillieul F, Manteau S, Audran JC, Haye B, Lambert B, Legendre L (1999) Differential induction of grapevine defenses by two strains of Botrytis cinerea. Phytopathology 89:197–203
Deunff EL, Davoine C, Dantec CL, Billard JP, Huault C (2004) Oxidative burst and expression of germin/O×O genes during wounding of ryegrass leaf blades: comparison with senescence of leaf sheaths. Plant J 38:421–431
Dutton MV, Evans CS (1996) Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment. Can J Microbiol 42:881–895
Fernández Acero FJ, Carbú M, El-Akhal MR, Garrido C, González-Rodríguez VE, Cantoral JM (2011) Development of proteomics-based fungicides: new strategies for environmentally friendly control of fungal plant diseases. Int J Mol Sci 12:795–816
Fernández-Acero FJ, Jorge I, Calvo E, Vallejo I, Carbu M, Camafeita E, Garrido C, Lopez JA, Jorrin J, Cantoral JM (2007) Proteomic analysis of phytopathogenic fungus Botrytis cinerea as a potential tool for identifying pathogenicity factors, therapeutic targets and for basic research. Arch Microbiol 187:207–215
Frías M, González M, González C, Brito N (2016) BcIEB1, a Botrytis cinerea secreted protein, elicits a defense response in plants. Plant Sci 250:115–124
Ghosh S, Narula K, Sinha A, Ghosh R, Jawa P, Chakraborty N, Chakraborty S (2016) Proteometabolomic analysis of transgenic tomato overexpressing oxalate decarboxylase uncovers novel proteins potentially involved in defense mechanism against Sclerotinia. J Proteomics 143:242–253
Godoy G, Steadman JR, Dickman MB, Dam R (1990) Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol Mol Plant Pathol 37:179–191
Hu X, Bidney DL, Yalpani N, Duvick JP, Crasta O, Folkerts O, Lu G-H (2003) Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol 133:170–181
Ikotun T (1984) Production of oxalic acid by Penicillium oxalicum in culture and in infected yam tissue and interaction with macerating enzyme. Mycopathologia 88:9–14
Kapat A, Zimand G, Elad Y (1998) Biosynthesis of pathogenicity hydrolytic enzymes by Botrytis cinerea during infection of bean leaves and in vitro. Mycol Res 102:1017–1024
Karmakar S, Molla KA, Chanda PK, Sarker SN, Datta SK, Datta K (2016) Green tissue-specific co-expression of chitinase and oxalate oxidase 4 genes in rice for enhanced resistance against sheath blight. Planta 243:115–130
Kesarwani M, Azam M, Natarajan K, Mehta A, Datta A (2000) Oxalate decarboxylase from Collybia velutipes molecular cloning and its overexpression to confer resistance to fungal infection in transgenic tobacco and tomato. J Biol Chem 275:7230–7238
Kotsira VP, Clonis YD (1997) Oxalate oxidase from barley roots: purification to homogeneity and study of some molecular, catalytic, and binding properties. Arch Biochem Biophys 340:239–249
Kumari S, Tayal P, Sharma E, Kapoor R (2014) Analyses of genetic and pathogenic variability among Botrytis cinerea isolates. Microbiol Res 169:862–872
Kurian P, Stelzig DA (1979) The synergistic role of oxalic acid and endopolygalacturonase in bean leaves infected by Cristulariella pyramidalis. Phytopathology 69:1301–1304
Lane BG (2000) Oxalate oxidases and differentiating surface structure in wheat: germins. Biochem J 349:309–321
Liang H, Maynard CA, Allen RD, Powell WA (2001) Increased Septoria musiva resistance in transgenic hybrid poplar leaves expressing a wheat oxalate oxidase gene. Plant Mol Biol 45:619–629
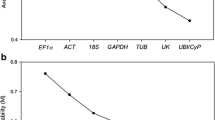
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Method Mol Biol 25:402–408
Lumsden RD (1979) Histology and physiology of pathogenesis in plant disease caused by Sclerotinia species. Phytopathology 69:890–896
Marciano P, Lenna PD, Magro P (1983) Oxalic acid, cell wall-degrading enzymes and pH in pathogenesis and their significance in the virulence of two Sclerotinia sclerotiorum isolates on sunflower. Physiol Mol Plant Pathol 22:339–345
Melotto M, Underwood W, He SY (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46:101–122
Molla KA, Karmakar S, Chanda PK, Ghosh S, Sarkar SN, Datta SK, Datta K (2013) Rice oxalate oxidase gene driven by green tissue-specific promoter increases tolerance to sheath blight pathogen (Rhizoctonia solani) in transgenic rice. Mol Plant Pathol 14:910–922
Munir F, Hayashi S, Batley J, Naqvi SMS, Mahmood T (2016) Germin-like protein 2 gene promoter from rice is responsive to fungal pathogens in transgenic potato plants. Funct Integr Genomic 16:19–27
Patykowski J, Urbanek H (2003) Activity of enzymes related to H2O2 generation and metabolism in leaf apoplastic fraction of tomato leaves infected with Botrytis cinerea. J Phytopathology 151:153–161
Rao DV, Tewari JP (1987) Production of oxalic acid by Mycena citricolor, causal agent of American leaf spot of coffee. Phytopathology 77:780–785
Rollins JA (2003) The Sclerotinia sclerotiorum Pac1 gene is required for sclerotial development and virulence. Mol Plant-Microbe Interact 16:785–795
Stone HE, Armentrout VN (1985) Production of oxalic acid by Sclerotium cepivorum during infection of onion. Mycologia 77:526–530
Walz A, Zingen-Sell I, Loeffler M, Sauer M (2008) Expression of an oxalate oxidase gene in tomato and severity of disease caused by Botrytis cinerea and Sclerotinia sclerotiorum. Plant Pathol 57:453–458
Wei Y, Zhang Z, Andersen CA, Schmelzer E, Gregersen PL, Collinge DB, Thordal-Christensen V, Smedegaard-Petersen H (1998) An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol Biol 36:101–112
Woo EJ, Dunwell JM, Goodenough PW, Marvier AC, Pickersgill RW (2000) Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat Struct Biol 7:1036–1040
Xu LS, Li GQ, Jiang DH, Chen WD (2018) Sclerotinia sclerotiorum: An evaluation of virulence theories. Annu Rev Phytopathol 56:311–338
Zhang Y, Wang C, Su P, Liao XL (2015) Control effect and possible mechanism of the natural compound phenazine-1-carboxamide against Botrytis cinerea. PLoS ONE 10:e0140380. https://doi.org/10.1371/journal.pone.0140380
Zhou F, Zhang Z, Gregersen PL, Mikkelsen JD, de Neergaard E, Collinge DB, Thordal-Christensen H (1998) Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiol 117:33–41
Zhu Y, Yu J, Brecht JK, Jiang T, Zheng X (2016) Pre-harvest application of oxalic acid increases quality and resistance to Penicillium expansum in kiwifruit during postharvest storage. Food Chem 190:537–543
Acknowledgements
We thank Prof. Brad Day (Michigan State University) for kindly revising the manuscript. This research was supported by the Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201303025), the 111 Project from the Education Ministry of China (Grant No. B07049), and by Northwest A&F University extention Project (2018–2010).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, G., Feng, C., Zhang, A. et al. The dual role of oxalic acid on the resistance of tomato against Botrytis cinerea. World J Microbiol Biotechnol 35, 36 (2019). https://doi.org/10.1007/s11274-019-2603-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2603-3