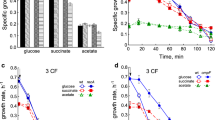
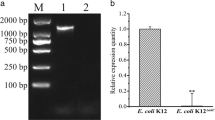
Abstract
Isothiazolones are used as preservatives in various modern industrial products. Although microorganisms that exhibit resistance towards these biocides have been identified, the underlying resistance mechanisms are still unclear. Therefore, we investigated the resistance properties of the following Burkholderia cepacia strains to Kathon (a representative of isothiazolones): a wild-type (WT) strain; a laboratory resistance strain (BC-IR) induced from WT; and an isolated strain (BC-327) screened from industrial contamination samples. The bacterial cell structure was disrupted by 50 μg ml−1 Kathon treatment. BC-IR and BC-327 did not display resistance in the presence of 1 ml L−1 Tween 80, 1 ml L−1 Triton X-100, 0.1 % sodium dodecyl sulfate or 1 mmol L−1 EDTA-2Na. Additionally, BC-IR and BC-327 exhibited lower relative conductivity from 10 to 180 min. The types as well as the levels of outer-membrane proteins (OMPs) were altered among WT, BC-IR and BC-327. Finally, the two Kathon-resistance strains BC-IR and BC-327 presented higher resistance capacity to H2O2. We measured the levels of peroxide-sensor genes and observed that the transcriptional activator oxyR, superoxide dismutase sod1, sod2, catalase cat1 and cat3 were all up-regulated under oxidative conditions for all strains. Taken together, OMPs and peroxide-sensor genes in B. cepacia contributed to isothiazolone resistance; However, the laboratory strain BC-IR exhibited a different resistance mechanism and properties compared to the isolated strain BC-327.





Similar content being viewed by others
References
Adibpour N, Khalaj A, Rezaee S, Daneshtalab M (2007) In vitro antifungal activity of 2-(4-substituted phenyl)-3(2H)-isothiazolones. Folia Microbiol 52:573–576
Adibpour N, Khalaj A, Rajabalian S (2010) Synthesis and antibacterial activity of isothiazolyl oxazolidinones and analogous 3(2H)-isothiazolones. Eur J Med Chem 45:19–24
Bogman K, Erne-Brand F, Alsenz J, Drewe J (2003) The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J Pharm Sci 92:1250–1261
Brözel VS, Cloete TE (1994) Resistance of Pseudomonas aeruginosa to isothiazolone. J Appl Bacteriol 76:576–582
Chapman JS, Diehl MA (1995) Methylchloroisothiazolone-induced growth inhibition and lethality in Escherichia coli. J Appl Microbiol 78:134–141
Chapman JS, Diehl MA, Fearnside KB (1998) Preservative tolerance and resistance. Int J Cosmet Sci 20:31–39
Cheng JJ, Thanassi JA, Thoma CL, Bradbury BJ, Deshpande M, Pucci MJ (2007) Dual targeting of DNA gyrase and topoisomerase IV: target interactions of heteroaryl isothiazolones in Staphylococcus aureus. Antimicrob Agents Chemother 51:2445–2453
Cloete TE (2003) Resistance mechanisms of bacteria to antimicrobial compounds. Int Biodeterior Biodegradation 51:277–282
Collier PJ, Austin P, Gilbert P (1990a) Uptake and distribution of some isothiazolone biocides into Escherichia coli ATCC 8739 and Schizosaccharomyces pombe NCYC 1354. Int J Pharm 66:201–206
Collier PJ, Ramsey AJ, Austin P, Gilbert P (1990b) Growth inhibitory and biocidal activity of some isothiazolone biocides. J Appl Microbiol 69:569–577
Collier PJ, Austin P, Gilbert P (1991) Isothiazolone biocides: enzyme-inhibiting pro-drugs. Int J Pharm 74:195–201
El Abdellaoui H, Varaprasad CVNS, Barawkar D, Chakravarty S, Maderna A, Tam R, Chen HM, Allan M, Wu JZ, Appleby T, Yan SQ, Zhang WJ, Lang S, Yao NH, Hamatake R, Hong Z (2006) Identification of isothiazole-4-carboxamidines derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg Med Chem Lett 16:5561–5566
Ettorre A, Andreassi M, Anselmi C, Neri P, Andreassi L, Di Stefano A (2003) Involvement of oxidative stress in apoptosis induced by a mixture of isothiazolinones in normal human keratinocytes. J Invest Dermatol 121:328–336
Furdas SD, Shekfeh S, Bissinger EM, Wagner JM, Schlimme S, Valkov V, Hendzel M, Jung M, Sippl W (2011) Synthesis and biological testing of novel pyridoisothiazolones as histone acetyltransferase inhibitors. Bioorgan Med Chem 19:3678–3689
Gedi V, Moon JY, Lim WM, Lee MY, Lee SC, Koo BS, Govindwar S, Yoon MY (2011) Identification and characterization of inhibitors of Haemophilus influenzae acetohydroxyacid synthase. Enzyme Microb Technol 49:1–5
Greenberg JT, Demple B (1986) Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol 168:1026–1029
Howard-Flanders P, Theriot L (1966) Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics 53:1137–1150
Jimenez L, Smalls S (2000) Molecular detection of Burkholderia cepacia in toiletry, cosmetic, and pharmaceutical raw materials and finished products. J AOAC Int 83:963–966
Khalaj A, Adibpour N, Shahverdi AR, Daneshtalab M (2004) Synthesis and antibacterial activity of 2-(4-substituted phenyl)-3(2H)-isothiazolones. Eur J Med Chem 39:699–705
King A, Lodola A, Carmi C, Fu J, Mor M, Piomelli D (2009) A critical cysteine residue in monoacylglycerol lipase is targeted by a new class of isothiazolinone-based enzyme inhibitors. Brit J Pharmacol 157:974–983
Kiselyov AS, Semenova M, Semenov VV (2009) 3, 4-Disubstituted isothiazoles: novel potent inhibitors of VEGF receptors 1 and 2. Bioorg Med Chem Lett 19:1195–1198
Leive L (1968) Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem 243:2373–2380
Lewin C, Doherty C, Govan J (1993) In vitro activities of meropenem, PD 127391, PD 131628, ceftazidime, chloramphenicol, co-trimoxazole, and ciprofloxacin against Pseudomonas cepacia. Antimicrob Agents Chemother 37:123–125
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods 25:402–408
Palleroni NJ (2005) Genus I. Burkholderia. In: Garrity GM (ed) Bergey's manual of systematic bacteriology, 2nd edn. Volume 2: The Proteobacteria, Part C: The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. Springer, New York, pp 575-600
Pedras MS, Suchy M (2006) Design, synthesis, and antifungal activity of inhibitors of brassilexin detoxification in the plant pathogenic fungus Leptosphaeria maculans. Bioorg Med Chem 14:714–723
Pucci MJ, Cheng JJ, Podos SD, Thoma CL, Thanassi JA, Buechter DD, Mushtaq G, Vigliotti GA, Bradbury BJ, Deshpande M (2007) In vitro and in vivo antibacterial activities of heteroaryl isothiazolones against resistant gram-positive pathogens. Antimicrob Agents Chemother 51:1259–1267
Pucci MJ, Podos SD, Thanassi JA, Leggio MJ, Bradbury BJ, Deshpande M (2011) In vitro and in vivo profiles of ACH-702, an isothiazoloquinolone, against bacterial pathogens. Antimicrob Agents Chemother 55:2860–2871
Reddy BM, Tanneeru K, Meetei PA, Guruprasad L (2011) 3D-QSAR and molecular docking studies on substituted isothiazole analogs as inhibitors against MEK-1 kinase. Chem Biol Drug Des 79:84–91
Rushton L, Sass A, Baldwin A, Dowson CG, Donoghue D, Mahenthiralingam E (2013) Key role for efflux in the preservative susceptibility and adaptive resistance of Burkholderia cepacia complex bacteria. Antimicrob Agents Chemother 57:2972–2980
Sharmeen L, McQuade T, Heldsinger A, Gogliotti R, Domagala J, Gracheck S (2001) Inhibition of the early phase of HIV replication by an isothiazolone, PD 161374. Antiviral Res 49:101–114
Speksnijder P, van Ravestijn J, de Voogt P (2010) Trace analysis of isothiazolinones in water samples by large-volume direct injection liquid chromatography tandem mass spectrometry. J Chromatogr A 1217:5184–5189
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tang QY, Feng MG (2007) DPS data processing system: experimental design, statistical analysis and data mining. Science Press, Beijing
Tattawasart U, Maillard JY, Furr JR, Russell AD (2000) Outer membrane changes in Pseudomonas stutzeri resistant to chlorhexidine diacetate and cetylpyridinium chloride. Int J Antimicrob Agents 16:233–238
Vermis K, Vandamme P, Nelis HJ (2003) Burkholderia cepacia complex genomovars: utilization of carbon sources, susceptibility to antimicrobial agents and growth on selective media. J Appl Microbiol 95:1191–1199
Vicentini CB, Romagnoli C, Manfredini S, Rossi D, Mares D (2011) Pyrazolo [3, 4-c]isothiazole and isothiazolo [4, 3-d]isoxazole derivatives as antifungal agents. Pharm Biol 49:545–552
Williams TM (2007) The mechanism of action of isothiazolone biocide. Power Plant Chem 9:14–22
Winder CL, Al-Adham IS, Abdel Malek SM, Buultjens TE, Horrocks AJ, Collier PJ (2000) Outer membrane protein shifts in biocide-resistant Pseudomonas aeruginosa PAO1. J Appl Microbiol 89:289–295
Woodcock D, Linsenmeyer M, Chojnowski G, Kriegler A, Nink V, Webster L, Sawyer W (1992) Reversal of multidrug resistance by surfactants. Br J Cancer 66:62
Xu FL, Lin Q, Hou BR (2009) Synthesis and bioactivity of novel benzisothiazolone derivatives as potential microbiocides. J Heterocycl Chem 46:320–323
Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M (1992) Proposal of Burkholderia gen. nov; and transfer of seven species of the Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol 36:1251–1275
Acknowledgments
This work was funded by Scientific and Technological Project of Guangdong Province (No. 2011B010500024), Strategic Cooperation Projects Guangdong Province and Chinese Academy (No. 2011B090300018) and Young Foundation of Guangdong Academy of Sciences for Scientific Research (No. qnjj201203).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, G., Shi, Qs., Ouyang, Ys. et al. Involvement of outer membrane proteins and peroxide-sensor genes in Burkholderia cepacia resistance to isothiazolone. World J Microbiol Biotechnol 30, 1251–1260 (2014). https://doi.org/10.1007/s11274-013-1538-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1538-3