Abstract
In this study, the major ginsenoside Rb1 was transformed into the more pharmacologically active minor compound K by food grade Lactobacillus paralimentarius LH4, which was isolated from kimchi, a traditional Korean fermented food. The enzymatic reaction was analyzed by TLC, HPLC, and NMR. Using the cell-free enzyme of Lactobacillus paralimentarius LH4 at optimal conditions for 30 °C at pH 6.0, 1.0 mg ml−1 ginsenoside Rb1 was transformed into 0.52 mg ml−1 compound K within 72 h, with a corresponding molar conversion yield of 88 %. The cell-free enzyme hydrolyzed the two glucose moieties attached to the C-3 position and the outer glucose moiety attached to the C-20 position of the ginsenoside Rb1. The cell-free enzyme hydrolyzed the ginsenoside Rb1 along the following pathway: ginsenoside Rb1 → gypenoside XVII and ginsenoside Rd → ginsenoside F2 → compound K. Our results indicate that Lactobacillus paralimentarius LH4 has the potential to be applied for the preparation of compound K in the food industry.
Similar content being viewed by others
Introduction
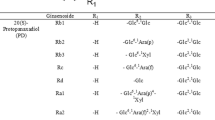
Ginseng, the root of Panax ginseng C. A. Meyer, belongs to the family Araliaceae. It has been used in Asia as a medicine to treat various diseases for several thousand years. Ginsenosides, the principal components of ginseng, are a class of triterpenoid saponins exhibiting diverse pharmacological activities such as anti-inflammatory (Wu et al. 1992), anti-tumor (Mochizuki et al. 1995), anti-fatigue (Lee et al. 2005), anti-diabetic (Ni et al. 2010), and anti-cancer activities (Chae et al. 2009). Currently, more than 180 ginsenosides have been discovered and identified in ginseng (Christensen 2008). The protopanaxadiol (PPD)-type ginsenosides are further classified into subgroups based on the position and number of sugar moieties attached to the aglycon at positions C-3 and C-20 (Fig. 1). Pharmaceutically active ginsenosides exist as deglycosylated forms at low concentrations or are absent in ginseng (Tawab et al. 2003). These deglycosylated ginsenosides, including ginsenosides Rg3, Rh2, and compound K, can be produced by the hydrolysis of sugar moieties from the major ginsenosides Rb1, Rb2, Rc, and Rd, accounting for more than 50 % of the total ginsenosides (Noh et al. 2009).
Several methods for the transformation of the major ginsenosides to the minor ginsenosides, including acid hydrolysis (Tawab et al. 2003), physical (Akao et al. 1988), alkali cleavage (Kim et al. 2000), and biological processes (Quan et al. 2010, 2011) have been previously attempted. However, most of the physical and chemical methods such as mild acid hydrolysis, chemical synthesis, and alkaline cleavage are accompanied by inevitable and undesirable side reactions (Keum et al. 2000). These problems of physical and chemical reactions could be avoided by using microbial or enzymatic conversion methods, as these methods use milder reaction conditions and are environmentally compatible.
Deglycosylated compound K, which is absent in ginseng root, reportedly induces tumor cell apoptosis, inhibits tumor metastasis, and restrains tumor invasion (Oh et al. 2004; Wakabayashi et al. 1997, 1998). Its production has been achieved from ginsenoside Rb1 using microbial methods, including the use of the crude enzyme from Caulobacter leidyia (Cheng et al. 2006), Fusarium sacchari (Han et al. 2007), and Acremonium strictum (Chen et al. 2008). However, these microorganisms are not food grade and are soil microorganisms and fungi, restricting their use in the food industry.
Kimchi, a traditional Korean fermented food, is a lactic-acid-fermented vegetable product consumed raw and is considered to be a good source of potentially beneficial and useful lactic acid bacteria. Kimchi microbiome is dominated by members of three genera: Leuconostoc, Lactobacillus, and Weissella (Jung et al. 2011). Among the 200 bacteria that have been isolated from kimchi, most of the bacteria belonging to the genus Lactobacillus have been found to be present in kimchi. In this study, we focused on the production of pharmacologically active minor compound K from major ginsenoside Rb1 using cell-free enzyme of food-grade Lactobacillus paralimentarius LH4 isolated from kimchi.
Materials and methods
Materials
Standard ginsenosides Rb1, Rd, F2, Rg3, Rh2, and compound K were obtained from the Ginseng Genetic Resource Bank (Kyung Hee University, Yongin, Korea).
Screening of lactic acid bacteria producing β-glucosidase
Esculin-MRS agar (Wang et al. 1983) was used to isolate β-glucosidase-producing lactic acid bacteria from home-made and commercial kimchi. The growth medium contained 3 g/l esculin with MRS agar. Lactic acid bacteria that produced β-glucosidase, which hydrolyzes esculin, appeared as colonies that were surrounded by a reddish-brown to dark brown zone on the esculin-MRS agar. Subsequent-ly, single colonies from those plates were subjected to additional incubation for 2 days at 37 °C in MRS broth.
Phylogenetic analysis
The phylogenetic relationship of strain LH4 to other bacteria was determined by analyzing a portion of the 16S rRNA gene. The 16S rRNA gene was first amplified by PCR using the universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) and then sequenced. A near-complete sequence of the 16S rRNA gene was compiled using the SeqMan program in the DNASTAR package. The 16S rRNA gene sequences of related taxa were obtained from GenBank (National Center for Biotechnology Information; Bethesda, MD, USA), and a phylogenetic tree was constructed via the neighbor-joining method using the MEGA 3.1 program. A bootstrap analysis with 1,000 replicates was conducted to obtain confidence levels for the branches. The closest strains were included in the phylogenetic tree.
Cultivation and biotransformation of the ginsenoside Rb1 and the enzyme assay
Lactobacillus paralimentarius LH4 was cultured in MRS broth at 30 °C for 12 h until its absorbance at 600 nm reached 1.0. Crude enzyme was obtained by centrifugation at 5,000×g for 40 min at 4 °C. For the biotransformation, ginsenoside Rb1 (1.0 mg ml−1) was dissolved in 20 mM sodium phosphate buffer (pH 6.0) and then reacted with crude enzyme (1 ml) at 30 °C for 72 h. During the reaction period, a 0.1 ml aliquot was taken every 24 h. An equal volume of water-saturated n-butanol was added to each sample to stop the reaction. The n-butanol fraction was then evaporated until dry, and the methanol extract was analyzed by TLC, HPLC, and NMR.
To determine the optimal pH for enzymatic activity, pNP-β-D-glucopyranoside hydrolyzing activity was studied at 30 °C in buffer at various pH (4–9). Fifty microliters of 10 mM pNP-β-D-glucopyranoside was added to 50 μl of crude cell extract and incubated at 30 °C for 30 min. The effect of temperature on enzymatic activity was tested by incubation of crude cell extract containing 10 mM pNP-β-D-glucopyranoside at various temperatures ranging from 25 to 60 °C for 30 min at the optimum pH. The reaction was stopped with 100 μl of 0.5 M Na2CO3, and the absorbance was measured at 405 nm. One unit of activity was defined as the amount of crude enzyme required to generate 1 μmol pNP per min.
TLC analysis of ginsenosides
TLC was performed with silica gel plates (60 F254, Merck, Darmstadt, Germany) with a developing solvent of CHCl3:CH3OH:H2O (65:35:10, v/v, lower phase). Spots on the TLC plates were detected by spraying the plates with 10 % H2SO4, followed by heating at 110 °C for 10 min.
Analysis of ginsenosides by HPLC and NMR
The reaction mixture was extracted with n-butanol saturated with H2O and evaporated under vacuum. The residue was dissolved in methanol and analyzed by HPLC using a C18 (250 × 4.6 mm, particle size 5 μm) column with acetonitrile (solvent A) and distilled water (solvent B) as the mobile phases at 85 % B for 5 min, 79 % B for 20 min, 42 % B for 55 min, 10 % B for 12 min, and 85 % B for 18 min at a flow rate of 1.6 ml min−1. Detection was at 203 nm. 13C-NMR spectra were obtained on a Bruker Av 600NMR spectrometer at 100 MHz with pyridine-d5 as the solvent.
Results and discussion
Phylogenetic study
The 16S rRNA gene sequence of strain LH4 was aligned with other strains found to have the closest taxonomic relationships. Strain LH4 was grouped with a Lactobacillus species, and the highest degree of 16S rRNA gene sequence identities were to Lactobacillus paralimentarius (99.8 %) (Fig. 2).
Biotransformation of ginsenoside Rb1 and structural identification of reaction products
The products of the hydrolysis of ginsenoside Rb1 by strain LH4 were determined at regular intervals by TLC analysis. As shown in Fig. 3, strain LH4 catalyzed the production of four different metabolites from ginsenoside Rb1. Based on a comparison of R f values with those of standard ginsenosides, metabolites 2, 3, and 4 were identified as ginsenosides Rd, F2, and compound K, respectively, while metabolite 1 was an unknown compound, as its R f value was different from known ginsenosides.
Metabolite 1 was therefore examined by NMR. In the 1H-NMR spectrum of metabolite 1, the proton signals for the H-1 of the 3-O-inner-glucopyranosyl moiety, 3-O-outer-glucopyranosyl moiety, and 20-glucopyranosyl moiety appeared at δ 4.89 ppm (1H, d, J = 8.0 Hz, H-3-glc-1H′), δ 5.03 ppm (1H, d, J = 7.6 Hz, H-20-glc-1H′″), and δ 5.08 ppm (1H, d, J = 7.6 Hz, H-20-glc-1H″″), respectively, showing that the aglycon of metabolite 1 harbored three β-D-glucoses. In the 13C NMR (pyridine-d5, 100 MHz) spectrum of metabolite 1, the signals for the C-2′ of the 3-inner-glucose was shifted up field, from 83.5 to 75.6 ppm, but the other signals were similar to those of ginsenoside Rb1. Compared with a previous report (Dong et al. 2003), we found that the metabolite is 3-O-[β-d-glucopyranosyl]-20-O-[β-d-glucopyranosyl-(6,1)-β-d-gluco-pyranosyl]-20(S)-protopanaxadiol, identical to gypenoside XVII (Table 1).
The conversion of ginsenoside Rb1 by strain LH4 was confirmed quantitatively by HPLC analysis (Fig. 4). It was found that 1 mg ml−1 ginsenoside Rb1 was transformed into 0.52 mg ml−1 compound K after 72 h, with a corresponding molar conversion yield of 88 %. Hence, strain LH4 hydrolyzed ginsenoside Rb1 along the pathway ginsenoside Rb1 → gypenoside XVII and ginsenoside Rd → ginsenoside F2 → compound K, with hydrolysis of the two glucose moieties attached to the C-3 position and the outer glucose attached to the C-20 position of ginsenoside Rb1 (Fig. 5). The crude enzyme from Lactobacillus pentosus DC101 exploits the hydrolytic pathway Rd → F2 → compound K (Quan et al. 2010), crude enzyme from Leuconostoc citreum LH1 exploits the hydrolytic pathway Rb1 → Rd → F2 → compound K (Quan et al. 2011), and crude cell extract of Lactobacillus delbrueckii exploits the hydrolytic pathway Rb1 → Rd → F2 → Rh2 (Chi and Ji 2005). As shown in Fig. 6, the maximal enzyme activity was determined to be at pH 6 and a temperature of 30 °C. Crude enzyme from Esteya vermicola CNU 120806 (Hou et al. 2012) and Leuconostoc mesenteroides 690 (Park et al. 2012) had optimal activity at pH 5 and 7 and temperatures of 50 and 37 °C, respectively.
Effect of pH (a) and temperature (b) on the activity of the crude enzyme using pNP-β-d-glucopyranoside as a substrate. The following buffers (20 mM) were tested: citric acid-sodium citrate buffer (pH 4–5), sodium phosphate buffer (pH 6–7), Tris–HCl buffer (pH 8–9). The effect of temperature on the enzyme activity was tested in 20 mM sodium phosphate buffer pH 6. Data represent the means of three experiments and error bars represent standard deviation
In conclusion, this study demonstrated that compound K was produced from the major ginsenoside Rb1 via the ginsenoside Rd, the gypenoside XVII, and the ginsenoside F2 by food-grade Lactobacillus paralimentarius LH4 isolated from kimchi. Strain LH4 completely converted ginsenoside Rb1 into the more pharmacologically active minor compound K with high productivity. Therefore, strain LH4 has the potential to be applied for the preparation of compound K in the food industry.
References
Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K (1988) Intestinal bacterial hydrolysis is required for the appearance of compound K in ratplasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol 50:1155–1160
Chae S, Kang KA, Chang WY, Kim MJ, Lee SJ, Lee YS, Kim HS, Kim DH, Hyun JW (2009) Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem 57:5777–5782
Chen GT, Yang M, Song Y, Lu ZQ, Zhang JQ, Huang HL, Wu LJ, Guo DA (2008) Microbial transformation of ginsenoside Rb1 by Acremonium strictum. Appl Microbiol Biotechnol 77:1345–1350
Cheng LQ, Kim MK, Lee JW, Lee YJ, Yang DC (2006) Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol Lett 28:1121–1127
Chi H, Ji GE (2005) Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett 27:765–771
Christensen LP (2008) Ginsenosides. Chemistry, biosynthesis, analysis and potential health effects. Adv Food Nutr Res 55:1–99
Dong A, Ye M, Guo H, Zheng J, Guo D (2003) Microbial transformation of ginsenoside Rb1 by Rhizopus stolonifer and Curvularia lunata. Biotechnol Lett 25:339–344
Han Y, Sun B, Hu X, Zhang H, Jiang B, Spranger MI, Zhao Y (2007) Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. J Agric Food Chem 55(23):9373–9379
Hou J, Xue J, Wang C, Liu L, Zhang D, Wang Z, Li W, Zheng Y, Sung C (2012) Microbial transformation of ginsenoside Rg3 to ginsenoside Rh2 by Esteya vermicola CNU 120806. World J Microbiol Biotechnol 28(4):1807–1811
Jung JY, Lee SH, Kim JM, Park MS, Bae JW, Hahn Y, Madsen EL, Jeon CO (2011) Metagenomic analysis of Kimchi, a traditional Korean fermented food. Appl Environ Microbiol 77:2264–2274
Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, Kwon H, Surh YJ (2000) Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett 150:41–48
Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH (2000) Steaming of ginseng at high temperature enhances biological activity. J Nat Prod 63:1702–1704
Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH (2005) Hepatoprotective effect of ginsenoside Rb1 and compound K on tertbutyl hydroperoxide-induced liver injury. Liver Int 25:1069–1073
Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S (1995) Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside Rb2, 20(R)- and 20(S)-ginsenoside Rg3, of Red ginseng. Biol Pharm Bull 18:1197–1202
Ni HX, Yu NJ, Yang XH (2010) The study of ginsenoside on PPAR gamma expression of mononuclear macrophage in type 2 diabetes. Mol Biol Rep 37:2975–2979
Noh KH, Son JW, Kim HJ, Oh DK (2009) Ginsenoside compound K production from ginseng root extract by a thermostable beta-glycosidase from Sulfolobussolfataricus. Biosci Biotechnol Biochem 73:316–321
Oh SH, Yin HQ, Lee BH (2004) Role of the Fas/Fas ligand death receptor pathway in ginseng saponin metabolite induced apoptosis in HepG2 cells. Arch Pharm Res 27:402–406
Park SJ, Youn SY, Ji GE, Park MS (2012) Whole cell biotransformation of major ginsenosides using Leuconostocs and Lactobacilli. Food Sci Biotechnol 21(3):839–844
Quan LH, Cheng LQ, Kim HB, Kim JH, Son NR, Kim SY, Jin HO, Yang DC (2010) Bioconversion of ginsenoside Rd into compound K by Lactobacillus pentosus DC101 isolated from Kimchi. J Ginseng Res 34:288–295
Quan LH, Piao JY, Min JW, Yang DU, Lee HN, Yang DC (2011) Bioconversion of ginsenoside Rb1 into compound K by Leuconostoc citreum LH1 isolated from Kimchi. Braz J Microbiol 42:1227–1237
Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M (2003) Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos 31:1065–1071
Wakabayashi C, Hasegawa H, Murata J, Saiki I (1997) In vivo anti metastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol Res 9:411–417
Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I (1998) An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun 246:725–730
Wang BX, Cui JC, Liu AJ, Wu SK (1983) Studies on the anti-fatigue effect of the saponins of stems and leaves of Panax ginseng (SSLG). J Tradit Chin Med 3:89–94
Wu JY, Gardner BH, Murphy CI, Seals JR, Kensil CR, Recchia J (1992) Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol 148:1519–1525
Acknowledgments
This research was supported by iPET (# 309019-3 & # 111035-3), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quan, LH., Kim, YJ., Li, G.H. et al. Microbial transformation of ginsenoside Rb1 to compound K by Lactobacillus paralimentarius . World J Microbiol Biotechnol 29, 1001–1007 (2013). https://doi.org/10.1007/s11274-013-1260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1260-1