Abstract
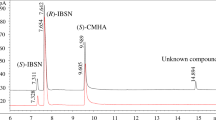
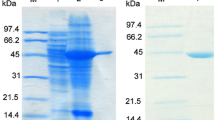
Amidase is a promising synthesis tool for chiral amides and related derivatives. In the present study, the biochemical properties of the Delftia tsuruhatensis CCTCC M 205114 enantioselective amidase were determined for its potential application in chiral amides synthesis. D. tsuruhatensis CCTCC M 205114 amidase was purified 105.2 fold with total activity recovery of 4.26%. The enzyme is a monomer with a subunit of approximately 50 kDa by analytical gel filtration HPLC and SDS–PAGE. It had a broad substrate spectrum and displayed high enantioselectivity against R-2, 2-dimethylcyclopropane carboxamide and R-mandelic amide. The amidase was applied to enantioselective hydrolysis of R-2, 2-dimethylcyclopropane carboxamide from racemic (R, S)-2, 2-dimethylcyclopropane carboxamide to accumulate S-2, 2-dimethylcyclopropane carboxamide. This enzyme did not require metal ions for the hydrolysis reaction. Its optimal pH and temperature were 8.0 and 35°C, respectively. The K m and V max of the amidase for R-2, 2-dimethylcyclopropane carboxamide were 2.54 mM and 8.37 μmol min−1 mg protein−1, respectively. After 60 min of the reaction, R-2, 2-dimethylcyclopropane carboxamide was completely hydrolyzed, generating S-2, 2-dimethylcyclopropane carboxamide with a yield of 45.9% and an e.e. of above 99%. Therefore, this amidase can serve as a promising producer for S-2, 2-dimethylcyclopropane carboxamide and other amides.





Similar content being viewed by others
References
Baek DH, Song JJ, Lee SG, Kwon SJ, Asano Y, Sung MH (2003) New thermostable d-methionine amidase from Brevibacillus borstelensis BCS-1 and its application for d-phenylalanine production. Enzyme Microb Technol 32:131–139
Banerjee A, Sharma R, Banerjee UC (2002) The nitrile-degrading enzymes: current status and future prospects. Appl Microbiol Biotechnol 60:33–44
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Briggs BS, Kreuzman AJ, Whitesitt C, Yeh WK, Zmijewski M (1996) Discovery, purification, and properties of O-phthalyl amidase from Xanthobacter agilis. J Mol Catal B Enzym 2:53–69
Chen J, Zheng RC, Zheng YG, Shen YC (2009) Microbial transformation of nitriles to high-value acids or amides. Adv Biochem Eng Biotechnol 113:33–77
Ciskanik LM, Wilczek JM, Fallon RD (1995) Purification and characterization of an enantioselective amidase from Pseudomonas chlororaphis B23. Appl Environ Microbiol 61:998–1003
d’Abusco AS, Ammendola S, Scandurra R, Politi L (2001) Molecular and biochemical characterization of the recombinant amidase from hyperthermophilic archaeon Sulfolobus solfataricus. Extremophiles 5:183–192
Doran JP, Duggan P, Masterson M, Turner PD, O’Reilly C (2005) Expression and purification of a recombinant enantioselective amidase. Protein Expr Purif 40:190–196
Egorova K, Trauthwein H, Verseck S, Antranikian G (2004) Purification and properties of an enantioselective and thermoactive amidase from the thermophilic actinomycete Pseudonocardia thermophila. Appl Microbiol Biotechnol 65:38–45
Gilligan T, Yamada H, Nagasawa T (1993) Production of S-(+)-2-phenylpropionic acid from (R, S)-2-phenylpropionitrile by the combination of nitrile hydratase and stereoselective amidase in Rhodococcus equi TG328. Appl Microbiol Biotechnol 39:720–725
Gopalakrishna KN, Stewart BH, Kneen MM, Andricopulo AD, Kenyon GL, McLeish MJ (2004) Mandelamide hydrolase from Pseudomonas putida: characterization of a new member of the amidase signature family. Biochemistry 43:7725–7735
Graham D, Pereira R, Barfield D, Cowan D (2000) Nitrile biotransformations using free and immobilized cells of a thermophilic Bacillus spp. Enzyme Microb Technol 26:368–373
Gregoriou M, Brown PR (1979) Inhibition of the aliphatic amidase from Pseudomonas aeruginosa by urea and related compounds. Eur J Biochem 96:101–108
Hayashi T, Yamamoto K, Matsuo A, Otsubo K, Muramatsu S, Matsuda A, Komatsu K (1997) Characterization and cloning of an enantioselective amidase from Comamonas acidovorans KPO-2771–4. J Ferment Bioeng 83:139–145
Hirrlinger B, Stolz A, Knackmuss HJ (1996) Purification and properties of an amidase from Rhodococcus erythropolis MP50 which enantioselectively hydrolyzes 2-arylpropionamides. J Bacteriol 178:3501–3507
Hongpattarakere T, Komeda H, Asano Y (2005) Purification, characterization, gene cloning and nucleotide sequencing of D-stereospecific amino acid amidase from soil bacterium: Delftia acidovorans. J Ind Microbiol Biotechnol 32:567–576
Jin SJ, Zheng RC, Zheng YG, Shen YC (2008) R-enantioselective hydrolysis of 2, 2-dimethylcyclopropanecarboxamide by amidase from a newly isolated strain Brevibacterium epidermidis ZJB-07021. J Appl Microbiol 105:1150–1157
Kagayama T, Ohe T (1990) Purification and properties of an aromatic amidase from Pseudomonas sp GDI-211. Agric Biol Chem 54:2565–2571
Kaplan A (1969) The determination of urea, ammonia and urease. Methods Biochem Anal 17:311–324
Kobayashi M, Shimizu S (2000) Nitrile hydrolases. Curr Opin Biotechnol 4:95–102
Komeda H, Asano Y (2000) Gene cloning, nucleotide sequencing, and purification and characterization of the D-stereospecific amino-acid amidase from Ochrobactrum anthropi SV3. Eur J Biochem 267:2028–2035
Komeda H, Harada H, Washika S, Sakamoto T, Ueda M, Asano Y (2004) A novel R-stereoselective amidase from Pseudomonas sp MCI3434 acting on piperazine-2-tert-butylcarboxamide. Eur J Biochem 271:1580–1590
Komeda H, Hariyama N, Asano Y (2006) L-stereoselective amino acid amidase with broad substrate specificity from Brevundimonas diminuta: Characterization of a new member of the leucine aminopeptidase family. Appl Microbiol Biotechnol 70:412–421
Kotlova EK, Chestukhina GG, Astaurova OB, Leonova TE, Yanenko AS, Debabov VG (1999) Isolation and primary characterization of an amidase from Rhodococcus rhodochrous. Biochemistry Mosc 64:384–389
Krieg L, Slusarczyk H, Verseck S, Kula MR (2005) Identification and characterization of a novel d-amidase gene from Variovorax paradoxus and its expression in Escherichia coli. Appl Microbiol Biotechnol 66:542–550
López-Serrano P, Wegman MA, van Rantwijk F, Sheldon RA (2001) Enantioselective enzyme catalyzed ammoniolysis of amino acid derivatives. Effect of temperature. Tetrahedron Asymmetr 12:235–240
Miyazawa T, Imagawa K, Yanagihara R, Yamada T (1997) Marked dependence on temperature of enantioselectivity in the Aspergillus oryzae protease-catalyzed hydrolysis of amino acid esters. Biotechnol Tech 11:931–933
Nawaz MS, Khan AA, Seng JE, Leakey JE, Siitonen PH, Cerniglia CE (1994) Purification and characterization of an amidase from an acrylamide-degrading Rhodococcus sp. Appl Environ Microbiol 60:3343–3348
Nawaz MS, Khan AA, Bhattacharayya D, Siitonen PH, Cerniglia CE (1996) Physical, biochemical, and immunological characterization of a thermostable amidase from Klebsiella pneumoniae NCTR 1. J Bacteriol 178(8):2397–2401
Park HJ, Uhm KN, Kim HK (2008) R-stereoselective amidase from Rhodococcus erythropolis no. 7 acting on 4-chloro-3-hydroxybutyramide. J Microbiol Biotechnol 18:552–559
Phillips RS (1996) Temperature modulation of the stereochemistry of enzymatic catalysis: Prospects for exploitation. Trends Biotechnol 14:13–16
Robins KT, Gilligan T (1994) Bacteria capable of stereospecifically hydrolyzing R-(−)-2,2-dimethylcyclopropanecarboxamide. US5360731
Sakai T (2004) ‘Low-temperature method’ for a dramatic improvement in enantioselectivity in lipase-catalyzed reactions. Tetrahedron Asymmetr 15:2749–2756
Sakai T, Kishimoto T, Tanaka Y, Ema T, Utaka M (1998) Low-temperature method for enhancement of enantioselectivity in the lipase-catalyzed kinetic resolutions of solketal and some chiral alcohols. Tetrahedron Lett 39:7881–7884
Suzuki Y, Ohta H (2006) Identification of a thermostable and enantioselective amidase from the thermoacidophilic archaeon Sulfolobus tokodaii strain 7. Protein Expr Purif 45:368–373
Wang YS, Xu JM, Zheng RC, Zheng YG, Shen YC (2008) Improvement of amidase production by a newly isolated Delftia tsuruhatensis ZJB-05174 through optimization of culture medium. J Microbiol Biotechnol 18:1932–1937
Wang YS, Zheng RC, Xu JM, Liu ZQ, Cheng F, Feng ZH, Liu LL, Zheng YG, Shen YC (2010) Enantioselective hydrolysis of (R)-2, 2-dimethylcyclopropane carboxamide by immobilized cells of an R-amidase-producing bacterium, Delftia tsuruhatensis CCTCC M 205114, on an alginate capsule carrier. J Ind Microbiol Biotechnol 37:503–510
Zheng RC, Wang YS, Liu ZQ, Xing LY, Zheng YG, Shen YC (2007a) Isolation and characterization of Delftia tsuruhatensis ZJB-05174, capable of R-enantioselective degradation of 2, 2-dimethylcyclopropanecarboxamide. Res Microbiol 158:258–264
Zheng RC, Zheng YG, Shen YC (2007b) Enantioseparation and determination of 2, 2-dimethylcyclopropanecarboxamide and corresponding acid in the bioconversion broth by gas chromatography. Biomed Chromatogr 21:610–615
Zimmermann T, Robins K, Birch OM, Bohlen E (1995) Genetic engineering process for the production of S-(+)-2,2-dimethylcyclopropanecarboxamide by microorganisms. US 5427934
Acknowledgments
We would like to thank Elise A. Lamonta (Department of Veterinary Population Medicine, University of Minnesota) for reviewing and valuable suggestions. This work was supported by the Major Basic Research Development Program of China (No. 2011CB710800) and the Natural Scientific Foundation of Zhejiang (Y407225 and Y4080334).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, YS., Cheng, F., Zheng, RC. et al. Characterization of an enantioselective amidase with potential application to asymmetric hydrolysis of (R, S)-2, 2-dimethylcyclopropane carboxamide. World J Microbiol Biotechnol 27, 2885–2892 (2011). https://doi.org/10.1007/s11274-011-0769-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0769-4