Abstract
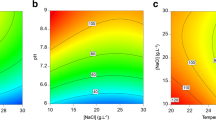
The red microalga Porphyridium contains many valuable compounds such as polysaccharides, polyunsaturated fatty acids, and phycoerythrin (PE). In this study, a uniform design method and regression analysis were used to investigate the effects of initial pH, light intensity, inoculation ratio, and liquid volume in flask on the optimal biomass, exopolysaccharides (EPS), and PE production of Porphyridium cruentum in a batch culture at laboratory scale. Using regression analysis, we obtained the models to clarify the effects of individual factors and their interactions on the biomass, EPS, and PE production of P. cruentum. The optimal condition for the biomass was the following: pH 5.0, light intensity 7098.0 lx, inoculation ratio 1:17.2, and liquid volume 100.0 ml; for EPS was pH 5.0, light intensity 4501.0 lx, inoculation ratio 1:20, and liquid volume 100.3 ml; while pH 8.0, light intensity 7100.0 lx, inoculation ratio 1:20, and liquid volume 100.3 ml was the best for PE production. The maximum biomass 3.27 g/l, EPS production 543.1 mg/l, and PE production 132.0 mg/l were demonstrated by confirmatory experiment to the optimum culture conditions in a reciprocal shaker. The statistical methods used in the present study are useful strategies for optimizing of culture conditions for other microalgae.
Similar content being viewed by others
References
Ayyagari MS, Pande R, Kanstekar S et al (1995) Molecular assembly of protein and conjugated polymers: toward development of biosensors. Biotechnol Bioeng 45:116–121
Benavides J, Rito-Palomares M (2006) Simplified two-stage method to B-phycoerythrin recovery from Porphyridium cruentum. J Chromatogr B, online
Bermejo R, Talavera EM, Alvarez-Pez JM (2001) Chromatographic purification and characterization of B-phycoerythrin from Porphyridium cruentum semipreparative high-performance liquid chromatographic separation and characterization of its subunits. J Chromatogr A 917:135–145
Cao Y, Zheng Y, Fang B (2004) Optimization of polymerase chain reaction-amplified conditions using the uniform design method. J Chem Technol Biotechnol 79:910–913
Cao Y, Xia Q, Fang B (2006) Optimization of expression of dhaT gene encoding 1,3-propanediol oxidoreductase from Klebsiella pneumoniae in Escherichia coli using the methods of uniform design and regression analysis. J Chem Technol Biotechnol 81:109–112
Chen XG, Li X, Kong L et al (2003) Application of uniform design and genetic algorithm in optimization of reversed-phase chromatographic separation. Chemometr Intell Lab Syst 67:157–166
Chen D, Han Y, Gu Z (2006) Application of statistical methodology to the optimization of fermentative medium for carotenoids production by Rhodobacter sphaieroides. Process Biochem 41:1773–1778
Dufossé L, Galaup P, Yaron A et al (2005) Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality? Trends Food Sci Tech 16:389–406
Fa’bregas J, Garcia D, Morales E et al (1998) Renewal rate of semicontinuous culture of the microalga Porphyridium cruentum modifies phycoerythrin, exopolysaccharide and fatty acid productivity. J Ferment Bioeng 86:477–481
Fang KT (1998) Uniform design and uniform design table. Science Press, Beijing, pp 1–5
Fang KT, Qin H (2003) A note on construction of nearly uniform designs with large number of runs. Stat Probab Lett 16:215–224
Huang J, Chen B, You W (2005) Studies on separation of extracellular polysaccharide of Porphyridium cruentum and its anti-HBV in vitro. Chin J Mar Drugs 24(5):18–21
Huleihel M, Ishanu V, Tal J et al (2001) Antiviral effect of red microalgal polysaccharides on herpes simplex and varicella zoster viruses. J Appl Phycol 13:127–134
Huleihel M, Ishanu V, Tal J et al (2002) Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J Biochem Biophys Methods 50:189–200
Iqbal M, Zafar SI (1993) Strategies toward optimization of cultural conditions of Porphyridium cruentum for higher polysaccharide production. Acta Microbiol Pol 42:71–82
Jones RE, Speer HL, Kury W (1963) Studies on the growth of the red alga Porphyridium cruentum. Physiol Plant 16:636–643
Liang YZ, Fang KT, Xu QS (2000) Uniform design and its applications in chemistry and chemical engineering. Chemometr Intell Lab Syst 53:21–35
Merchuk JC, Ronen M, Giris S et al (1998) Light/dark cycles in the growth of the red microalga Porphyridium sp. Biotechnol Bioeng 59:705–713
Muller-Feuga A, Gue´des RL, Pruvost J (2003) Benefits and limitations of modeling for optimization of Porphyridium cruentum cultures in an annular photobioreactor. J Biotechnol 103:153–163
Nuutila MA, Aura AM, Kiesvaara M (1997) The effect of salinity, nitrate concentration, pH and temperature on eicosapentaenoic acid (EPA) production by the red unicellular alga Porphyridium purpureum. J Biotechnol 55:55–63
Sarada R, Bhattacharya S, Ravishankar GA (2002) Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J Microbiol Biotechnol 18:517–521
Singh S, Arad (Malis) S, Richmond A (2000) Extracellular polysaccharide production in outdoor mass cultures of Porphyridium sp. in flat plate glass reactors. J Appl Phycol 12:269–275
Tetzuya K (1988) Phycobilisome stability. Meth Enzymol 167:313–318
Wang J, Chen B, Wang M et al (2004) Effect of culture conditions on the growth and metabolites production of Porphyridium cruentum. J Fujian Norm Univ 20(3):63–66
Wen S, Zhang T, Tan T (2005) Optimization of the amino acid composition in glutathione fermentation. Process Biochem 40:3474–3479
You T, Barnett ST (2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J 19:251–258
Zhang W (1999) Biochemical technique of glycoconjugates. Zhejiang University Press, Hangzhou, pp 11–12
Acknowledgments
This study was funded by Development and Reform Commission of Fujian Province of China [Minji(2003)203] and the Natural Science Foundation of Fujian Province of China (No. B0410008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Chen, B., Rao, X. et al. Optimization of culturing conditions of Porphyridium cruentum using uniform design. World J Microbiol Biotechnol 23, 1345–1350 (2007). https://doi.org/10.1007/s11274-007-9369-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9369-8