Summary
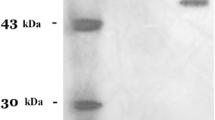
An extracellular naringinase (an enzyme complex consisting of α-L-rhamnosidase and β-D-glucosidase activity, EC 3.2.1.40) that hydrolyses naringin (a trihydroxy flavonoid) for the production of rhamnose and glucose was purified from the culture filtrate of Aspergillus niger 1344. The enzyme was purified 38-fold by ammonium sulphate precipitation, ion exchange and gel filtration chromatography with an overall recovery of 19% with a specific activity of 867 units per mg of protein. The molecular mass of the purified enzyme was estimated to be about 168 kDa by gel filtration chromatography on a Sephadex G-200 column and the molecular mass of the subunits was estimated to be 85 kDa by sodium dodecyl sulphate-Polyacrylamide gel electrophoresis (SDS-PAGE). The enzyme had an optimum pH of 4.0 and temperature of 50 °C, respectively. The naringinase was stable at 37 °C for 72 h, whereas at 40 °C the enzyme showed 50% inactivation after 96 h of incubation. Hg2+, SDS, p-chloromercuribenzoate, Cu2+ and Mn2+ completely inhibited the enzyme activity at a concentration of 2.5–10 mM, whereas, Ca2+, Co2+ and Mg2+ showed very little inactivation even at high concentrations (10–100 mM). The enzyme activity was strongly inhibited by rhamnose, the end product of naringin hydrolysis. The enzyme activity was accelerated by Mg2+ and remained stable for one year after storage at −20 °C. The purified enzyme preparation successfully hydrolysed naringin and rutin, but not hesperidin.
Similar content being viewed by others
References
R. Bourbouze F. Percheron J. Courtois (1976) ArticleTitleα-L-Rhamnosidase de Fagopyrum esculentum European Journal of Biochemistry 248 953–956
C. Caldini F. Bonomi P. Pifferi G. Lanzarani Y. Galente (1994) ArticleTitleKinetic and immobilization studies on fungal glycosidase for aroma enhancement in wine Enzyme and Microbial Technology 16 286–291 Occurrence Handle10.1016/0141-0229(94)90168-6
B. Chandler K. Nicol (1975) ArticleTitleSome relationships of naringin: their importance in orange juice bitterness CSIRO Food Research Quarterly 35 79–88
L. Daniels R. Linhardt B. Bryan F. Mayerl M. Pickenhagen (1990) ArticleTitleMethods for producing rhamnose US Patent 4 933, 281
D.W. Davis (1947) ArticleTitleDetermination of flavonones in citrus juice Analytical Chemistry 19 46–48
M.V. Gallego F. Pinaga D. Ramon S. Valles (2001) ArticleTitlePurification and characterization of an α-L-rhamnosidase from Aspergillus terreus of interest in wine making Food Toxicology 66 204–209
K. Habelt F. Pittner (1983) ArticleTitleA rapid method for the determination of naringin, pruning, and naringenin applied to the assay of naringinase Analytical Biochemistry 134 393–397 Occurrence Handle6418025
S. Hasegawa V.P. Maier (1993) ArticleTitleSolution to the limonin bitterness problem of citrus juices Food Technology 37 73–77
W. Hashimoto K. Murata (1998) ArticleTitleα-L-Rhamnosidase of Sphingomonas sp. R1 producing an unusual exoploysaccharide of sphingan Bioscience Biotechnology and Biochemistry 62 1068–1074
I.S. Jang D.H. Kim (1996) ArticleTitlePurification and characterization of an α-L-rhamnosidase from Bacteroides JY-6, a human intestinal bacterium Biological and Pharmaceutical Bulletin 19 1546–1549 Occurrence Handle8996636
R.L. Johnson B.V. Chandler (1988) ArticleTitleAdsorptive removal of bitter principles and titrable acids from citrus juices Food Technology 45 130–137
D.A. Kimball (1991) Citrus Processing: Quality Control Technology Van Nostrand Reinhold NY
Y. Kurosawa K. Ikeda F. Egami (1973) ArticleTitleα-L-Rhamnosidase of the liver of Turbo cornutus Journal of Biochemistry 73 31–37 Occurrence Handle4632197
U.K. Laemmli (1970) ArticleTitleCleavage of structural proteins during the assembly of the head of the bacteriophage T Nature 227 680–685 Occurrence Handle5432063
A. Manjon J. Bastida C. Romero A. Jimeno J.L. Iborra (1985) ArticleTitleImmobilization of naringinase on glycophase coated porous glass beads Biotechnology Letters 7 487–492
P. Manzanares L.H. deGraff J. Visser (1997) ArticleTitlePurification and characterization of an α-L-rhamnosidase from Aspergillus niger FEMS Microbiology Letters 15 279–283
M. Mutter G. Beldman H.A. Schols A.G. Voragen (1994) ArticleTitleRhamnogalactouronan α-L-rhamnopyranohydrolase. A novel enzyme specific for the terminal nonreducing rhamnosyl unit in rhamnogalacturonan regions of pectin Plant Physiology 106 241–250 Occurrence Handle7972516
M. Ono T. Tosa I. Chibata (1978) ArticleTitlePreparation and properties of immobilized naringinase using tannin-aminohexylcellulose Agricultural and Biological Chemistry 42 1847–1853
M. Puri S.S. Marwaha R.M. Kothari (1996) ArticleTitleComparative kinetic characterization of soluble and alginate entrapped naringinase Enzyme and Microbial Technology 18 281–285
M. Puri U.C. Banerjee (2000) ArticleTitleProduction, purification and characterization of the debittering enzyme naringinase Biotechnology Advances 18 207–217 Occurrence Handle14538108
M. Puri M. Seth S.S. Marwaha R.M. Kothari (2001) ArticleTitleDebittering of kinnow mandarin juice by covalently immobilized naringinase on hen egg white Food Biotechnology 15 15–27
Puri M., Banerjee A., Banerjee U.C. 2004. Studies on the optimization of process parameters for the production of naringinase by Aspergillus niger MTCC1344 Process Biochemistry (in press).
C. Romero A. Manjon J. Bastida J.L. Iborra (1985) ArticleTitleA method for assaying rhamnosidase activity of naringinase Analytical Biochemistry 149 566–571 Occurrence Handle3935009
M.A. Sanchez C. Romero A. Manjon J.L. Iborra (1987) ArticleTitleActivity of soluble and immobilized hesperidinase on insoluble hesperidin Biotechnology Letters 9 871–874
Sankyo (1988) ArticleTitlePreparation of antibiotic chloropolysporin C Japanese Patent 63 146–797
D.W. Thomas C.V. Smythe M.D. Labbee (1958) ArticleTitleEnzymatic hydrolysis of naringin, the bitter principle of grapefruit Food Research 23 591–598
T. Yanai M. Sato (2000) ArticleTitlePurification and characterization of an α-L-rhamnosidase from Pichia angusta X349 Bioscience Biotechnology and Biochemistry 64 2179–2185
N.M. Young R.A. Johnston J.C. Richards (1989) ArticleTitlePurification of the α-L-rhamnosidase of Penicillum decumbens and characterization of two glycopeptide components Carbohydrate Research 191 53–62
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puri, M., Kalra, S. Purification and characterization of naringinase from a newly isolated strain of Aspergillus niger 1344 for the transformation of flavonoids. World J Microbiol Biotechnol 21, 753–758 (2005). https://doi.org/10.1007/s11274-004-5488-7
Issue Date:
DOI: https://doi.org/10.1007/s11274-004-5488-7