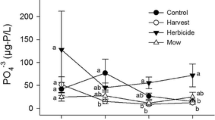
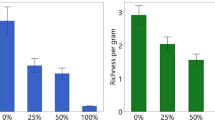
Abstract
Invasive common reed (Phragmites australis) can rapidly form expansive, near-monotypic stands, and thereby lower plant diversity and change marsh habitat structure. Consequently, North American wetland managers often use herbicides, such as glyphosate-based AquaNeat® and imazypr-based Habitat®, to control its establishment and spread. However, herbiciding might indirectly affect benthic community structure by directly altering habitat structure, and habitat alterations may vary with herbicide and concentration. These effects may be particularly pronounced ≥1 year post-herbiciding when dead above-ground biomass collapses and submerges. To evaluate how herbicide-caused alterations in habitat affect key trophic linkages, we compared snail and epiphytic algal assemblages, and habitat conditions, among 20- × 20-m replicated plots of reed treated with either AquaNeat® (30 % solution), Habitat® (5 % solution), or left herbicide-free (i.e., controls) in an eutrophic Lake Erie coastal marsh 1-y post-herbiciding. Both herbicides equally reduced reed above-ground growth by >90 % relative to controls. Fossaria spp. and Gyraulus parvus snails were more abundant in herbicide-treated plots than in controls, but Shannon-Wiener diversity was similar (H′ ≈ 1.0) across treatments. All snails collected were pulmonates, suggesting habitat drying might be driving assemblage structure. Snails were denser in plots with metaphyton (mostly Spirogyra) than without, and metaphyton was more abundant in herbicide-treated plots with higher incident light levels and warmer water temperatures than in controls. Snail biomass was positively related to amount of benthic macro-organic matter but not epiphytic algal biomass, which was similar among treatments. Diatoms dominated algal communities in all treatments. In June, Navicula spp. was dominant in controls, whereas Nitzschia palea and Aulacoseira italic, and Nitzschia spp., were dominant in AquaNeat® and Habitat® treatments, respectively. However, algal and diatom assemblages were similar in treatments by early-July when marsh water levels significantly decreased and nitrate levels were <1 μg/L. Marsh hydrologic patterns may mediate herbiciding’s indirect effects on trophic structure.







Similar content being viewed by others
References
Ágoston-Szabó E, Dinka M (2008) Decomposition of Typha angustifolia and Phragmites australis in the littoral zone of a shallow lake. Biologia 63:1104–1110
Ailstock MS, Norman CM, Bushmann PJ (2001) Common reed Phragmites australis: control and effects upon biodiversity in freshwater nontidal wetlands. Restor Ecol 9:49–59
Albay M, Akcaalan R (2003) Comparative study of periphyton colonisation on common reed (Phragmites australis) and artificial substrate in a shallow lake, Manyas, Turkey. Hydrobiologia 506:531–540
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington DC
Armstrong J, Afreen-Zobayed F, Armstrong W (1996) Phragmites die-back: sulphide- and acetic acid-induced bud and root death, lignifications, and blockages with aeration and vascular systems. New Phytol 134:601–614
Aufderheide J, Warbritton R, Pounds N, File-Emperador S, Staples C, Caspers N, Forbes V (2006) Effects of husbandry parameters on the life history traits of the apple snail Marisa cornuarietis: effects of temperature, photoperiod, and population density. Invertebr Biol 125:9–20
Auld JR (2008) Implications of size-selective predation and mate availability for mating-system expression and evolution in a hermaphroditic snail (Physa acuta). PhD Dissertation, University of Pittsburgh, Pittsburgh, Pennsylvania
Back CL, Holomuzk JR (2008) Long-term herbicide control of invasive, common reed (Phragmites australis) at Sheldon Marsh, Lake Erie. Ohio J Sci 108:108–112
Barnes V (2003) Identification and control of common reed (Phragmites australis) in Virginia. Virginia Cooperative Extension Fact Sheet number 427-101. Virginia State University. http://www.ext.vt.edu/pubs/weeds/427-101/427-101.html
Bickel TO, Closs GP (2009) Impact of partial removal of the invasive macrophyte Lagarosiphon major (Hydrocharitacea) on invertebrates and fish. River Res Appl 25:734–744
Borchardt MA (1996) Nutrients. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 184–227
Brown KM (1982) Resource overlap and competition in pond snails: an experimental analysis. Ecology 63:412–422
Burkholder JM (1996) Interactions of benthic algae with their substrata. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 253–297
Byers JE (2000) Competition between two estuarine snails: implications for invasions of exotic species. Ecology 81:1225–1239
Caffrey JM (1996) Glyphosate in fisheries management. Hydrobiologia 340:259–263
Carter VP, Rybicki NB, Habberschlag R (1991) Effects of submersed macrophytes on dissolved oxygen, pH, and temperature under different conditions of wind, tide, and bed structure. J Freshw Ecol 6:121–133
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64:261–273
Cornell LP, Klarer DM (2008) Patterns of dissolved oxygen, productivity and respiration in Old Woman Creek Estuary, Erie County, Ohio during low and high water conditions. Ohio J Sci 108:31–43
Daldorph PWG, Thomas JD (1991) Evaluation of bioengineering approaches aimed at controlling pulmonate snails: the effect of light attenuation and mechanical removal of macrophytes. J Appl Ecol 28:532–546
DeNicola DM (1996) Periphyton responses to temperature at different ecological levels. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic systems. Academic Press, San Diego, pp 150–183
Entrix Inc. (2003) Ecological risk assessment of the proposed use of the herbicide imazapyr to control invasive cordgrass (Spartina spp.) in estuarine habitat of Washington State. Prepared for Washington State of Agriculture. Project No. 3000901
Fell PE, Warren RS, Light JK, Rawson L Jr, Fairley SM (2003) Comparison of fish and macroinvertebrate use of Typha angustifolia, Phragmites australis, and treated Phragmites marshes along the lower Connecticut River. Estuaries 26:534–551
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup herbicide. Rev Environ Contam Toxicol 167:35–120
Gosselain V, Hudson G, Cattaneo A, Gagnon P, Planas D, Rochefort D (2005) Physical variables driving epiphytic algal biomass in a dense macrophyte bed of the St. Lawrence River (Quebec, Canada). Hydrobiologia 534:11–24
Holomuzki JR, Klarer DM (2010) Invasive reed effects on benthic community structure in Lake Erie coastal marshes. Wetlands Ecol Manage 18:219–231
Holomuzki JR, Short TM (1988) Habitat use and fish avoidance behaviors by the stream-dwelling isopod Lirceus fontinalis. Oikos 52:79–86
Hutchens JJ Jr, Walters K (2006) Gastropod abundance and biomass relationships with salt marsh vegetation within ocean-dominated South Carolina, USA estuaries. J Shellfish Res 25:947–953
Jigyasu HV, Singh VK (2010) Effect of environmental factors on the fecundity, hatchability and survival of snail Lymnaea (Radix) acuminata (Lamarck): vector of fascioliasis. J Water Health 8:109–115
Kesler DH, Jokinen EH, Munns WR Jr (1986) Trophic preferences and feeding morphology of two pulmonate snail species from a small New England pond, U.S.A. Can J Zool 64:2570–2575
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasser flora von Mitteleuropa, Band 2/1. Gustav Fischer, Stuttgart, New York, pp 1–876
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/2. VEB Gustav Fischer, Jena, pp 1–610
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D. (eds) Süsswasserflora von Mitteleuropa, Band 2/3. Gustav Fischer, Stuttgart, Jena, pp 1–598
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis Teil 1-4. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/4. Gustav Fischer, Stuttgart, Jena, pp 1–437
Kulesza AE, Holomuzki JR (2006) Amphipod performance responses to decaying leaf litter of Phragmites australis and Typha angustifolia from a Lake Erie coastal marsh. Wetlands 26:1079–1088
Kulesza AE, Holomuzki JR, Klarer DM (2008) Benthic community structure in stands of Typha angustifolia and herbicide-treated and untreated Phragmites australis. Wetlands 28:40–56
Květ J, Westlake DF (1998) Primary production in wetlands. In: Westlake DF, Květ J, Szczepański A (eds) The production ecology of wetlands. Cambridge University Press, Cambridge, pp 78–268
Lacki MJ, Hummer JW, Webster HJ (1990) Diversity patterns of invertebrate fauna in cattail wetlands receiving acid mine drainage. In: Skousen J, Scencindiver J, Samuel D (eds) Proceedings, 1990 mining and reclamation conference and exhibition, vol II, pp 365–371
Laugaste R, Reunanen M (2005) The composition and density of epiphyton on some macrophyte species in the partly meromictic Lake Verevi. Hydrobiologia 547:137–150
Lodge DM, Brown KM, Klosiewski SP (1987) Distribution of freshwater snails: spatial scale and the relative importance of physiochemical and biotic factors. Am Malacol Bull 5:73–84
Marks M, Lapin B, Randall J (1994) Phragmites australis (P. communis): threats, management, and monitoring. Nat Areas J 14:285–294
McCune B, Mefford MJ (2006) PC-ORD. Multivariate analysis of ecological data. Version 5. MjM Software Design, Gleneden Beach, Oregon
Morang A, Chader S (2005) Geology and historical evolution of Sheldon Marsh Nature Preserve, Lake Erie, Ohio. US Army Corps of Engineers, Engineer Research and Development Center, Coastal and Hydraulics Laboratory, Vicksburg
Mozdzer TJ, Hutto CJ, Clarke PA, Field DP (2008) Efficacy of imazapyr and glyphosate in the control of non-native Phragmites australis. Restor Ecol 16:221–224
Mundy CJ, Hann BJ (1996) Snail-periphyton interactions in a praire wetland. University Field Station, Delta Marsh, Annual report 31:40–52
Peckarsky BL, Fraissinet PR, Penton MA, Conklin DJ Jr (1990) Freshwater macroinvertebrates of northeastern North America. Cornell University Press, Ithica
Pennak RW (1989) Fresh-water invertebrates of the United States: protozoa to mollusca, 3rd edn. Wiley-Interscience, New York
Prescott GW (1982) Algae of the western Great Lakes area. Otto Koeltz Science Publishers, Koenigstein
Raichel DL, Able KW, Hartman JM (2003) The influence of Phragmites (Common Reed) on the distribution, abundance, and potential prey of a resident marsh fish in the Hackensack Meadowlands, New Jersey. Estuaries 26:511–521
Saltonstall K (2001) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99:2445–2449
Scott JT, Doyle RD, Filstrup CT (2005) Periphyton nutrient limitation and nitrogen fixation potential along a wetland nutrient-depletion gradient. Wetlands 25:439–448
Shannon CE, Weiner W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Stewart TW, Downing JA (2008) Macroinvertebrate communities and environmental conditions in recently constructed wetlands. Wetlands 28:141–150
Taft CE, Taft CW (1971) The algae of western Lake Erie. Bull Ohio biol survey. Ohio State University, Columbus, OH
Templer P, Findlay S, Wigand C (1998) Sediment chemistry associated with native and non-native emergent macrophytes of a Hudson River marsh ecosystem. Wetlands 18:70–78
Trexel-Kroll D (2002) Succession of floating-leaf to emergent plant communities following reduced water levels in Old Woman Creek Estuary. MSc Thesis, Miami University, Oxford, Ohio
USEPA (2003) Office of pesticide programs. Work plan for the registration of conventional pesticides. http://www.epa.gov/opprd001/workplan/fy2003workplan
Warren RS, Fell PE, Grimsby JL, Buck EL, Rilling GC, Fertik RA (2001) Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental Phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River. Estuaries 24:90–107
Wehr JD, Sheath RG (2003) Freshwater Algae of North America: ecology and classification. Academic Press, New York
Weinsten MP, Balletto JH (1999) Does the common reed, Phragmites australis, affect essential fish habitat. Estuaries 22:793–802
Wells FE (2008) The relationship between environmental variables and the density of the mudsnail Hydrobia totteni in a Nova Scotia salt marsh. J Molluscan Studies 74:355–362
Whitcraft CR, Levin LA (2007) Regulation of benthic algal and animal communities by salt marsh plants: impacts of shading. Ecology 88:904–917
Whyte RS, Holomuzki JR, Klarer DM (2009) Wetland plant and macroinvertebrate recovery in Phragmites australis-dominated stands after herbicide (Habitat®) treatment. Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie 30:725–730
Wilkinson L (2000) SYSTAT 9. SPSS, Chicago
Acknowledgments
We thank Steve Harvey and Mike Grote (ODNR, Division of Natural Areas and Preserves) for applying herbicides to experimental plots, Alex Wyatt for assistance collecting field data, and Frank Lopez of the Old Woman Creek National Estuarine Research Reserve for use of the dormitory. The manuscript benefited greatly from comments from Stu Ludsin, Tom Watters, Douglas Wilcox, and an anonymous reviewer. This work was supported in part by a contract to JRH through award NA09NOS4190080 from the Oceanic and Atmospheric Administration, administered by the Ohio Department of Natural Areas and Preserves through the Ohio Coastal Management Program, and was done in partial fulfillment of requirements for a MSc. Degree at The Ohio State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Back, C.L., Holomuzki, J.R., Klarer, D.M. et al. Herbiciding invasive reed: indirect effects on habitat conditions and snail–algal assemblages one year post-application. Wetlands Ecol Manage 20, 419–431 (2012). https://doi.org/10.1007/s11273-012-9265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-012-9265-3