Abstract
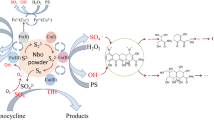
In this study, nano-calcium peroxide (nCP) was synthesized successfully and characterized by SEM, TEM, XRD, EDS, and BET techniques. The nCP was confirmed in nano-size with good dispersion, large specific surface area, high purity, and performed better than commercial calcium peroxide (CP) in naphthalene (NAP) removal when catalyzed by Fe(II). At the nCP/Fe(II)/NAP molar ratio of 15/20/1, 81.6% of NAP was degraded within 180 min, and this value was increased to 95.4% at the nCP/Fe(II)/citric acid (CA)/NAP molar ratio of 15/20/4/1 under the same conditions, suggesting that CA could effectively improve NAP removal. Further experiments were conducted to investigate the effects of reagents (nCP, Fe(II), and CA) dosage on NAP degradation. The results of probe experiments, electro-spin resonance spectrometer (EPR) detection and scavenging tests confirmed the presence of HO•, O2−•, and 1O2 in the nCP/Fe(II)/CA system, and HO• was the dominant radical for NAP degradation. According to the intermediates detected by GC–MS, three possible NAP degradation pathways were postulated. Finally, the NAP degradation under different water matrixes (pH, Cl−, and HCO3−) were explored, and the achievement of 97.7% removal of NAP in actual groundwater demonstrated that the nCP/Fe(II)/CA system has great potential for the remediation of NAP-contaminated groundwater.
Graphical abstract











Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Abdel-Shafy, H. I., & Mansour, M. S. M. (2016). A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum, 25, 107–123. https://doi.org/10.1016/j.ejpe.2015.03.011
Ahmad, M., Teel, A. L., Furman, O. S., Reed, J. I., & Watts, R. J. (2012). Oxidative and reductive pathways in iron-ethylenediaminetetraacetic acid–activated persulfate systems. Journal of Environmental Engineering, 138, 411–418. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000496
Ali, M., Danish, M., Tariq, M., Ahmad, A., Shahzad, A. K., & Lyu, S. (2020). Mechanistic insights into the degradation of trichloroethylene by controlled release nano calcium peroxide activated by iron species coupled with nano iron sulfide. Chemical Engineering Journal, 399, 125754. https://doi.org/10.1016/j.cej.2020.125754
Ali, M., Zhang, X., Idrees, A., Tariq, M., Danish, M., Farooq, U., Shan, A., Jiang, X., Huang, J., & Lyu, S. (2021). Advancement in Fenton-like reactions using PVA coated calcium peroxide/FeS system: Pivotal role of sulfide ion in regenerating the Fe(II) ions and improving trichloroethylene degradation. Journal of Environmental Chemical Engineering, 9, 104591. https://doi.org/10.1016/j.jece.2020.104591
Babuponnusami, A., & Muthukumar, K. (2014). A review on Fenton and improvements to the Fenton process for wastewater treatment. Journal of Environmental Chemical Engineering, 2, 557–572. https://doi.org/10.1016/j.jece.2013.10.011
Berkani, M., Vasseghian, Y., Le, V. T., Dragoi, E.-N., & Mousavi Khaneghah, A. (2021). The Fenton-like reaction for Arsenic removal from groundwater: Health risk assessment. Environmental Research, 202, 111698. https://doi.org/10.1016/j.envres.2021.111698
Buxton, G. V., Greenstock, C. L., Helman, W. P., & Ross, A. B. (1988). Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. Journal of Physical and Chemical Reference Data, 17, 513–886. https://doi.org/10.1063/1.555805
Chen, R., & Pignatello, J. J. (1997). Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environmental Science & Technology, 31, 2399–2406. https://doi.org/10.1021/es9610646
Deng, Y., & Zhao, R. (2015). Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Current Pollution Reports, 1, 167–176. https://doi.org/10.4018/978-1-5225-5766-1
Draganić, Z. D., Negrón-Mendoza, A., Sehested, K., Vujošević, S. I., Navarro-Gonzáles, R., Albarrán-Sanchez, M. G., & Draganić, I. G. (1991). Radiolysis of aqueous solutions of ammonium bicarbonate over a large dose range. International Journal of Radiation Applications and Instrumentation. Part c. Radiation Physics and Chemistry, 38, 317–321. https://doi.org/10.1016/1359-0197(91)90100-G
Field, T. B., McCourt, J. L., & McBryde, W. A. E. (1974). Composition and stability of iron and copper citrate complexes in aqueous solution. Canadian Journal of Chemistry, 52, 3119–3124. https://doi.org/10.1139/v74-458
García-Martínez, M. J., Canoira, L., Blázquez, G., Da Riva, I., Alcántara, R., & Llamas, J. F. (2005). Continuous photodegradation of naphthalene in water catalyzed by TiO2 supported on glass Raschig rings. Chemical Engineering Journal, 110, 123–128. https://doi.org/10.1016/j.cej.2005.03.020
Gu, M., Sui, Q., Farooq, U., Zhang, X., Qiu, Z., & Lyu, S. (2018). Degradation of phenanthrene in sulfate radical based oxidative environment by nZVI-PDA functionalized rGO catalyst. Chemical Engineering Journal, 354, 541–552. https://doi.org/10.1016/j.cej.2018.08.039
Huie, R. E., & Clifton, C. L. (1990). Temperature dependence of the rate constants for reactions of the sulfate radical, SO4−, with anions. The Journal of Physical Chemistry, 94, 8561–8567. https://doi.org/10.1021/j100386a015
Ji, Y., Wang, L., Jiang, M., Lu, J., Ferronato, C., & Chovelon, J. (2017). The role of nitrite in sulfate radical-based degradation of phenolic compounds: An unexpected nitration process relevant to groundwater remediation by in-situ chemical oxidation (ISCO). Water Research, 123, 249–257. https://doi.org/10.1016/j.watres.2017.06.081
Jiang, Y., Chen, Z., Li, M., Xiang, Q., Wang, X., Miao, H., & Ruan, W. (2021). Degradation of diclofenac sodium using Fenton-like technology based on nano-calcium peroxide. Science of the Total Environment, 773, 144801. https://doi.org/10.1016/j.scitotenv.2020.144801
Kaewdee, P., Chandet, N., Rujijanagul, G., & Randorn, C. (2016). Multicatalytic properties of nanoparticle CaO2 synthesized by a novel, simple and economical method for wastewater treatment. Catalysis Communications, 84, 151–154. https://doi.org/10.1016/j.catcom.2016.06.031
Khodaveisi, J., Banejad, H., Afkhami, A., Olyaie, E., Lashgari, S., & Dashti, R. (2011). Synthesis of calcium peroxide nanoparticles as an innovative reagent for in situ chemical oxidation. Journal of Hazardous Materials, 192, 1437–1440. https://doi.org/10.1016/j.jhazmat.2011.06.060
Kwon, B. G., Lee, D. S., Kang, N., & Yoon, J. (1999). Characteristics of p-chlorophenol oxidation by Fenton’s reagent. Water Research, 33, 2110–2118. https://doi.org/10.1016/S0043-1354(98)00428-X
Lawal, A. T. (2017). Polycyclic aromatic hydrocarbons A Review. Cogent Environmental Science, 3, 1339841. https://doi.org/10.1080/23311843.2017.1339841
Li, Y., Sun, J., & Sun, S. (2016). Mn2+-mediated homogeneous Fenton-like reaction of Fe(III)-NTA complex for efficient degradation of organic contaminants under neutral conditions. Journal of Hazardous Materials, 313, 193–200. https://doi.org/10.1016/j.jhazmat.2016.04.003
Li, Z., Sun, Y., Yang, Y., Han, Y., Wang, T., Chen, J., & Tsang, D. C. W. (2020). Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. Journal of Hazardous Materials, 383, 121240. https://doi.org/10.1016/j.jhazmat.2019.121240
Liang, C., & Su, H. (2009). Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Industrial & Engineering Chemistry Research, 48, 5558–5562. https://doi.org/10.1021/ie9002848
Madan, S. S., Wasewar, K. L., & Ravi, K. C. (2016). Adsorption kinetics, thermodynamics, and equilibrium of α-toluic acid onto calcium peroxide nanoparticles. Advanced Powder Technology, 27, 2112–2120. https://doi.org/10.1016/j.apt.2016.07.024
Mojiri, A., Zhou, J. L., Ohashi, A., Ozaki, N., & Kindaichi, T. (2019). Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Science of the Total EnvironmeNt, 696, 133971. https://doi.org/10.1016/j.scitotenv.2019.133971
Nardello, V., Aubry, J., Briviba, K., & Sies, H. (1998). Identification of the precursor of singlet oxygen (1O2, 1Δg) involved in the disproportionation of hydrogen peroxide catalyzed by calcium hydroxide. Chemical Communications, 5, 599–600. https://doi.org/10.1039/A708161H
Neta, P., Huie, R. E., & Ross, A. B. (1988). Rate constants for reactions of inorganic radicals in aqueous solution. Journal of Physical and Chemical Reference Data, 17, 1027–1284. https://doi.org/10.1063/1.555808
Northup, A., & Cassidy, D. (2008). Calcium peroxide (CaO2) for use in modified Fenton chemistry. Journal of Hazardous Materials, 152, 1164–1170. https://doi.org/10.1016/j.jhazmat.2007.07.096
Onwudili, J. A., & Williams, P. T. (2007). Reaction mechanisms for the decomposition of phenanthrene and naphthalene under hydrothermal conditions. The Journal of Supercritical Fluids, 39, 399–408. https://doi.org/10.1016/j.supflu.2006.03.014
Pan, Y., Su, H., Zhu, Y., Vafaei, M. H., & Long, M. (2018). CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals. Water Research, 145, 731–740. https://doi.org/10.1016/j.watres.2018.09.020
Pignatello, J. J. (1992). Dark and photoassisted iron (3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environmental Science & Technology, 26, 944–951. https://doi.org/10.1021/es00029a012
Preuss, R., Angerer, J., & Drexler, H. (2003). Naphthalene—an environmental and occupational toxicant. International Archives of Occupational and Environmental Health, 76, 556–576. https://doi.org/10.1007/s00420-003-0458-1
Ross, F., & Ross, A. B. (1977). Selected specific rates of reactions of transients from water in aqueous solution III Hydroxyl radical and perhydroxyl radical and their radical ions. Nat Stand Ref Data Ser Nat Bur Stand (U.S.) NSRDS-NBS, 59, 122. https://doi.org/10.2172/6635906
So, H. L., Chu, W., & Wang, Y. H. (2019). Naphthalene degradation by Fe2+/Oxone/UV – applying an unconventional kinetics model and studying the reaction mechanism. Chemosphere, 218, 110–118. https://doi.org/10.1016/j.chemosphere.2018.11.091
Stuglik, Z., & PawełZagórski, Z. (1981). Pulse radiolysis of neutral iron(II) solutions: Oxidation of ferrous ions by OH radicals. Radiation Physics and Chemistry, 1977(17), 229–233. https://doi.org/10.1016/0146-5724(81)90336-8
Sun, Y., Danish, M., Ali, M., Shan, A., Li, M., Lyu, Y., Qiu, Z., Sui, Q., Zang, X., & Lyu, S. (2020). Trichloroethene degradation by nanoscale CaO2 activated with Fe(II)/FeS: The role of FeS and the synergistic activation mechanism of Fe(II)/FeS. Chemical Engineering Journal, 394, 124830. https://doi.org/10.1016/j.cej.2020.124830
Sun, Y., Lyu, S., Brusseau, M. L., Tang, P., Jiang, W., Gu, M., Li, M., Lyu, Y., Qiu, Z., & Sui, Q. (2019). Degradation of trichloroethylene in aqueous solution by nanoscale calcium peroxide in the Fe(II)-based catalytic environments. Separation and Purification Technology, 226, 13–21. https://doi.org/10.1016/j.seppur.2019.05.075
Teel, A. L., & Watts, R. J. (2002). Degradation of carbon tetrachloride by modified Fenton’s reagent. Journal of Hazardous Materials, 94, 179–189. https://doi.org/10.1016/S0304-3894(02)00068-7
Venny, S. G., & Ng, H. K. (2012). Modified Fenton oxidation of polycyclic aromatic hydrocarbon (PAH)-contaminated soils and the potential of bioremediation as post-treatment. Science of the Total Environment, 419, 240–249. https://doi.org/10.1016/j.scitotenv.2011.12.053
Walling, C. (1975). Fenton’s reagent revisited. Accounts of Chemical Research, 8, 125–131. https://doi.org/10.1021/ar50088a003
Wang, H., Zhao, Y., Li, T., Chen, Z., Wang, Y., & Qin, C. (2016). Properties of calcium peroxide for release of hydrogen peroxide and oxygen: A kinetics study. Chemical Engineering Journal, 303, 450–457. https://doi.org/10.1016/j.cej.2016.05.123
Wilkinson, F., Helman, W. P., & Ross, A. B. (1995). Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. Journal of Physical and Chemical Reference Data, 24, 663–677. https://doi.org/10.1063/1.555965
Wu, C., & Linden, K. G. (2010). Phototransformation of selected organophosphorus pesticides: Roles of hydroxyl and carbonate radicals. Water Research, 44, 3585–3594. https://doi.org/10.1016/j.watres.2010.04.011
Xu, X., Li, X., Li, X., & Li, H. (2009). Degradation of melatonin by UV, UV/H2O2, Fe2+/H2O2 and UV/Fe2+/H2O2 processes. Separation and Purification Technology, 68, 261–266. https://doi.org/10.1016/j.seppur.2009.05.013
Xue, Y., Gu, X., Lu, S., Miao, Z., Brusseau, M. L., Xu, M., Fu, X., Zhang, X., Qiu, Z., & Sui, Q. (2016). The destruction of benzene by calcium peroxide activated with Fe(II) in water. Chemical Engineering Journal, 302, 187–193. https://doi.org/10.1016/j.cej.2016.05.016
Xue, Y., Lu, S., Fu, X., Sharma, V. K., Mendoza-Sanchez, I., Qiu, Z., & Sui, Q. (2018). Simultaneous removal of benzene, toluene, ethylbenzene and xylene (BTEX) by CaO2 based Fenton system: Enhanced degradation by chelating agents. Chemical Engineering Journal, 331, 255–264. https://doi.org/10.1016/j.cej.2017.08.099
Yang, X., Cai, H., Bao, M., Yu, J., Lu, J., & Li, Y. (2018). Insight into the highly efficient degradation of PAHs in water over graphene oxide/Ag3PO4 composites under visible light irradiation. Chemical Engineering Journal, 334, 355–376. https://doi.org/10.1016/j.cej.2017.09.104
Yang, R., Zeng, G., Xu, Z., Zhou, Z., Huang, J., Fu, R., & Lyu, S. (2021). Comparison of naphthalene removal performance using H2O2, sodium percarbonate and calcium peroxide oxidants activated by ferrous ions and degradation mechanism. Chemosphere, 283, 131209. https://doi.org/10.1016/j.chemosphere.2021.131209
Yuan, D., Zhang, C., Tang, S., Li, X., Tang, J., Rao, Y., Wang, Z., & Zhang, Q. (2019). Enhancing CaO2 Fenton-like process by Fe(II)-oxalic acid complexation for organic wastewater treatment. Water Research, 163, 114861. https://doi.org/10.1016/j.watres.2019.114861
Zhang, Q., He, D., Li, X., Feng, W., Lyu, C., & Zhang, Y. (2020a). Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. Journal of Hazardous Materials, 384, 121350. https://doi.org/10.1016/j.jhazmat.2019.121350
Zhang, T., Liu, Y., Zhong, S., & Zhang, L. (2020b). AOPs-based remediation of petroleum hydrocarbons-contaminated soils: Efficiency, influencing factors and environmental impacts. Chemosphere, 246, 125726. https://doi.org/10.1016/j.chemosphere.2019.125726
Zhang, X., Gu, X., Lu, S., Miao, Z., Xu, M., Fu, X., Qiu, Z., & Sui, Q. (2015). Degradation of trichloroethylene in aqueous solution by calcium peroxide activated with ferrous ion. Journal of Hazardous Materials, 284, 253–260. https://doi.org/10.1016/j.jhazmat.2014.11.030
Zhang, Y., & Zhou, M. (2019). A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. Journal of Hazardous Materials, 362, 436–450. https://doi.org/10.1016/j.jhazmat.2018.09.035
Zhou, Y., Fang, X., Wang, T., Hu, Y., & Lu, J. (2017). Chelating agents enhanced CaO2 oxidation of bisphenol A catalyzed by Fe3+ and reuse of ferric sludge as a source of catalyst. Chemical Engineering Journal, 313, 638–645. https://doi.org/10.1016/j.cej.2016.09.111
Funding
This study was financially supported by a grant from the National Natural Science Foundation of China (No. 41977164) and “One Belt and One Road” International Academic Cooperation and Exchange Program of Shanghai Science and Technology Committee (No. 19230742200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Sheng, X., Zhou, Z. et al. Insight into Naphthalene Degradation by Nano-calcium Peroxide in Fe(II)-Citric Acid Catalytic Environment. Water Air Soil Pollut 232, 503 (2021). https://doi.org/10.1007/s11270-021-05457-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05457-w