Abstract
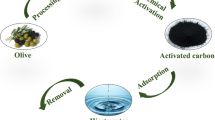
Olive pomace (OP) is an agricultural by-product of the olive oil production process. Olive oil mills generate a huge amount of OP that has adverse impacts on the environment due to its phytotoxic properties. Therefore, the utilization of OP in the production of activated carbon may be considered as an alternative eco-friendly solution for disposal and recycling of this waste. The adsorption of sulfadiazine (SDZ), an antibiotic of the sulfonamide group, using activated carbon from olive pomace (OPAC) was investigated under different experimental conditions. The characterization of synthesized activated carbon samples was performed by elemental analysis, BET surface area, total pore volume, average pore size, pHpzc, FTIR, and SEM-EDX analysis. In batch experiments, the effects of adsorption parameters such as pH of the solution, contact time, activated carbon dosage, initial SDZ concentration, and temperature were assessed. The results showed that the pH of the solution slightly affected the SDZ adsorption capacity of OPAC. In order to express the adsorption behavior of SDZ onto OPAC, Langmuir, Freundlich, Dubinin–Radushkevich (D–R), and Temkin isotherm models were applied to the experimental equilibrium data. The maximum adsorption capacity calculated from the Langmuir isotherm was 66.2252 mg/g at 298 K. The data from kinetic studies were analyzed using pseudo-first-order and pseudo-second-order models. The results indicated that the adsorption process of SDZ onto OPAC followed the pseudo-second-order model rather than the pseudo-first-order model. Based on the thermodynamic parameters including ΔG0, ∆H0, and ∆S0, it can be concluded that the nature of the adsorption process is exothermic, spontaneous, and favorable and of increased disorder and randomness. This work demonstrates that OP can be used as a precursor for activated carbon production and may substitute with commercial activated carbon for the removal of antibiotics from aqueous media. The production of activated carbon from OP will provide both minimization and recovery of this waste.














Similar content being viewed by others
References
Abdel-Ghani, N. T., El-Chaghaby, G. A., El-Gammal, M. H., & Rawash, E. A. (2016). Optimizing the preparation conditions of activated carbons from olive cake using KOH activation. New Carbon Materials, 31(5), 492–500.
Ahmaruzzaman, M., & Gayatri, S. L. (2010). Batch adsorption of 4-nitrophenol by acid activated jute stick char: equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal, 158, 173–180.
Ahmed, M. B., Zhou, J. L., Ngo, H. H., & Guo, W. (2015). Adsorptive removal of antibiotics from water and wastewater: progress and challenges. Science of the Total Environment, 532, 112–126.
Ahmed, M. B., Zhou, J. L., Ngo, H. H., Guo, W., Johir, M. A. H., & Sornalingam, K. (2017). Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chemical Engineering Journal, 311, 348–358.
Aksu, Z., & Dönmez, G. (2003). A comparative study on the biosorption characteristics of some yeasts for Remazol blue reactive dye. Chemosphere, 50, 1075–1083.
Alberti, G., Amendola, V., Pesavento, M., & Biesuz, R. (2012). Beyond the synthesis of novel solid phases: review on modelling of sorption phenomena. Coordination Chemistry Reviews, 256, 28–45.
Aljundi, I. H., & Jarrah, N. (2008). A study of characteristics of activated carbon produced from Jordanian olive cake. Journal of Analytical and Applied Pyrolysis, 81(1), 33–36.
Anastopoulos, I., & Kyzas, G. Z. (2016). Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? Journal of Molecular Liquids, 218, 174–185.
Annab, H., Fiol, N., Villaescusa, I., & Essamri, A. (2019). A proposal for the sustainable treatment and valorisation of olive mill wastes. Journal of Environmental Chemical Engineering, 7, 102803.
ASTM D2866-94. (2004). Standard test method for total ash content of activated carbon. West Conshohocken: ASTM International www.astm.org.
ASTM D2867-99. (1999). Standard test methods for moisture in activated carbon. West Conshohocken: ASTM International www.astm.org.
ASTM D5832-98. (2014). Standard test method for volatile matter content of activated carbon samples. West Conshohocken: ASTM International www.astm.org.
Azhar, M. R., Abid, H. R., Sun, H., Periasamy, V., Tadé, M. O., & Wang, S. (2016). Excellent performance of copper based metal organic framework in adsorptive removal of toxic sulfonamide antibiotics from wastewater. Journal of Colloid and Interface Science, 478, 344–352.
Azhar, M. R., Abid, H. R., Periasamy, V., Sun, H., Tadé, M. O., & Wang, S. (2017). Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. Journal of Colloid and Interface Science, 500, 88–95.
Baccar, R., Blánquez, P., Bouzid, J., Feki, M., Attiya, H., & Sarrà, M. (2013). Modeling of adsorption isotherms and kinetics of a tannery dye onto an activated carbon prepared from an agricultural by-product. Fuel Processing Technology, 106, 408–415.
Banerjee, S., & Chattopadhyaya, M. C. (2017). Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arabian Journal of Chemistry, 10, S1629–S1638.
Bao, X., Qiang, Z., Chang, J. H., Ben, W., & Qu, J. (2014). Synthesis of carbon-coated magnetic nanocomposite (Fe3O4@C) and its application for sulfonamide antibiotics removal from water. Journal of Environmental Sciences, 26, 962–969.
Bártíková, H., Podlipná, R., & Skálov, L. (2016). Veterinary drugs in the environment and their toxicity to plants. Chemosphere, 144, 2290–2301.
Basu, A., Behera, S. S., Dash, S., Banerjee, S., Sarkar, S., Mohanty, C. K., et al. (2019). A study on removal of Cr (III) from aqueous solution using biomass of Cymbopogon flexuosus immobilized in sodium alginate beads and its use as hydrogenation catalyst. Journal of the Taiwan Institute of Chemical Engineers, 102, 118–132.
Bello, O. S., Alao, O. C., Alagbada, T. C., & Olatunde, A. M. (2019). Biosorption of ibuprofen using functionalized bean husks. Sustainable Chemistry and Pharmacy, 13, 100151.
Białk-Bielińska, A., Stolte, S., Arning, J., Uebers, U., Böschen, A., Stepnowski, P., et al. (2011). Ecotoxicity evaluation of selected sulfonamides. Chemosphere, 85, 928–933.
Bohli, T., Fiol, N., Villaescusa, I., & Ouederni, A. (2013). Adsorption on activated carbon from olive stones: kinetics and equilibrium of phenol removal from aqueous solution. Journal of Chemical Engineering & Process Technology, 4(6), 1–5.
Bohli, T., Ouederni, A., Fiol, N., & Villaescusa, I. (2015). Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. C. R. Chimie, 18, 88–99.
Boparai, H. K., Joseph, M., & O’Carroll, D. M. (2011). Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. Journal of Hazardous Materials, 186, 458–465.
Bouchelta, C., Medjram, M. S., Bertrand, O., & Bellat, J. P. (2008). Preparation and characterization of activated carbon from date stones by physical activation with steam. Journal of Analytical and Applied Pyrolysis, 82, 70–77.
Bouki, C., Venieri, D., & Diamadopoulos, E. (2013). Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: a review. Ecotoxicology and Environmental Safety, 91, 1–9.
Braschi, I., Blasioli, S., Gigli, L., Gessa, C. E., Alberti, A., & Martucci, A. (2010). Removal of sulfonamide antibiotics from water: evidence of adsorption into an organophilic zeolite Y by its structural modifications. Journal of Hazardous Materials, 178, 218–225.
Candido, I. C. M., Soares, J. M. D., Barbosa, J. A. B., & Oliveira, H. P. (2019). Adsorption and identification of traces of dyes in aqueous solutions using chemically modified eggshell membranes. Bioresource Technology Reports, 7, 100267.
Chen, J., & Xie, S. (2018). Overview of sulfonamide biodegradation and the relevant pathways and microorganisms. Science of the Total Environment, 640-641, 1465–1477.
Chen, R., Guo, L., & Xu, S. (2014). Experimental and theoretical investigation of 1-hydroxybenzotriazole as a corrosion inhibitor for mild steel in sulfuric acid medium. International Journal of Electrochemical Science, 9, 6880–6895.
Chunhui, Z., Liangliang, W., Xiangyu, G., & Xudan, H. (2016). Antibiotics in WWTP discharge into the Chaobai River, Beijing. Archives of Environmental Protection, 42(4), 48–57.
Conde-Cid, M., Fernández-Calviño, D., Nóvoa-Muñoz, J. C., Arias-Estévez, M., Díaz-Raviña, M., Núñez-Delgado, A., et al. (2018). Degradation of sulfadiazine, sulfachloropyridazine and sulfamethazine in aqueous media. Journal of Environmental Management, 228, 239–248.
Cui, C., Jin, L., Jiang, L., Han, Q., Lin, K., Lu, S., et al. (2016). Removal of trace level amounts of twelve sulfonamides from drinking water by UV-activated peroxymonosulfate. Science of the Total Environment, 572, 244–251.
Ćurković, L., Ašperger, D., Babić, S., & Župan, J. (2018). Adsorption of enrofloxacin onto natural zeolite: kinetics, thermodynamics, isotherms and error analysis. Indian Journal of Chemical Technology, 25, 565–571.
Dai, Y., Li, J., & Shan, D. (2020). Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere, 238, 124432.
Demiral, H., Demiral, İ., Tümsek, F., & Karabacakoğlu, B. (2008). Adsorption of chromium (VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chemical Engineering Journal, 144, 188–196.
Du, L., & Liu, W. (2012). Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agronomy for Sustainable Development, 32, 309–327.
Dubinin, M. M., & Radushkevich, L. V. (1947). The equation of the characteristic curve of activated charcoal. Proceedings of the Academy of Sciences, Physical Chemistry Section, 55, 331–337.
El Hanandeh, A., Abu-Zurayk, R. A., Hamadneh, I., & Al-Dujaili, A. H. (2016). Characterization of biochar prepared from slow pyrolysis of Jordanian olive oil processing solid waste and adsorption efficiency of Hg2+ ions in aqueous solutions. Water Science & Technology, 74(8), 1899–1910.
Elmouwahidi, A., Bailón-García, E., Pérez-Cadenas, A. F., Maldonado-Hódar, F. J., & Carrasco-Marín, F. (2017). Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochimica Acta, 229, 219–228.
Elmouwahidi, A., Castelo-Quibén, J., Vivo-Vilches, J. F., Pérez-Cadenas, A. F., Maldonado-Hódar, F. J., & Carrasco-Marín, F. (2018). Activated carbons from agricultural waste solvothermally doped with sulphur as electrodes for supercapacitors. Chemical Engineering Journal, 334, 1835–1841.
Eroglu, H., Yapici, S., Nuhoglu, C., & Varoglu, E. (2009). Biosorption of Ga-67 radionuclides from aqueous solutions onto waste pomace of an olive oil factory. Journal of Hazardous Materials, 172, 729–738.
Faria, P., Órfão, J., & Pereira, M. (2004). Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Research, 38, 2043–2052.
Freundlich, H. (1906). Adsorption in solution. Physical Chemistry Society, 40, 1361–1368.
García-Galán, M. J., Díaz-Cruz, M. S., & Barcelo, D. (2008). Identification and determination of metabolites and degradation products of sulfonamide antibiotics. Trends in Analytical Chemistry, 27, 1008–1022.
Gaspard, S., Passé-Coutrin, N., Durimel, A., Cesaire, T., & Jeanne-Rose, V. (2014). Activated carbon from biomass for water treatment, chapter 2, In Sarra Gaspard and Mohamed Chaker Ncibi (Ed.), Biomass for sustainable applications: pollution remediation and energy (pp 46–105), UK: The Royal Society of Chemistry, RSC Green Chemistry Series, No.25, RSC Publishing.
Ghouma, I., Jeguirim, M., Dorge, S., Limousy, L., Ghimbeu, C. M., & Ouederni, A. (2015). Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. C. R. Chimie, 18, 63–74.
Gokulakumar, B., & Narayanaswamy, R. (2008). Fourier transform-infrared spectra (FT-IR) analysis of toot rot disease in sesame (Sesamum indicum). Romanian Journal of Biophysics, 8(3), 217–223.
Grenni, P., Ancona, V., & Caracciolo, A. B. (2018). Ecological effects of antibiotics on natural ecosystems: a review. Microchemical Journal, 136, 25–39.
Guo, Z., Zhou, F., Zhao, Y., Zhang, C., Liu, F., Bao, C., et al. (2012). Gamma irradiation-induced sulfadiazine degradation and its removal mechanisms. Chemical Engineering Journal, 191, 256–262.
Hadoun, H., Sadaoui, Z., Souami, N., Sahel, D., & Toumert, I. (2013). Characterization of mesoporous carbon prepared from date stems by H3PO4 chemical activation. Applied Surface Science, 280, 1–7.
Hazzaa, R., & Hussein, M. (2015). Adsorption of cationic dye from aqueous solution onto activated carbon prepared from olive stones. Environmental Technology & Innovation, 4, 36–51.
Hema, M., & Arivoli, S. (2007). Comparative study on the adsorption kinetics and thermodynamics of dyes onto acid activated low cost carbon. International Journal of Physical Sciences, 2(1), 010–017.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Hu, Q., & Zhang, Z. (2019). Application of Dubinin–Radushkevich isotherm model at the solid/ solution interface: a theoretical analysis. Journal of Molecular Liquids, 277, 646–648.
Jiang, M., Yang, W., Zhang, Z., Yang, Z., & Wang, Y. (2015). Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. Journal of Environmental Sciences, 31, 226–234.
Karthikeyan, P., Banu, H. A. T., & Meenakshi, S. (2019). Removal of phosphate and nitrate ions from aqueous solution using La3+ incorporated chitosan biopolymeric matrix membrane. International Journal of Biological Macromolecules, 124, 492–504.
Kosasih, A. N., Febrianto, J., Sunarso, J., Ju, Y. H., Indraswati, N., & Ismadji, S. (2010). Sequestering of Cu (II) from aqueous solution using cassava peel (Manihot esculenta). Journal of Hazardous Materials, 180, 366–374.
Kumar, R. R., Lee, J. T., & Cho, J. Y. (2012). Fate, occurrence, and toxicity of veterinary antibiotics in environment. Journal of the Korean Society for Applied Biological Chemistry, 55, 701–709.
Kumari, L. U., Beegum, M. F., Harikumar, B., Varghese, H. T., & Panicker, C. Y. (2008). Vibrational spectroscopic studies and AB initio calculations of 4-tert butyl benzyl selenocyanate. Rasāyan Journal of Chemistry, 1(1), 110–116.
Kümmerer, K. (2001). Drugs in the environment: emissions of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere, 45, 957–969.
Kümmerer, K. (2009). The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. Journal of Environmental Management, 90, 2354–2366.
Lagergren, S. (1898). Zur theorie der sogenannten adsorption gelösterstoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar, 24(4), 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica, and platinum. Journal of the American Chemistry Society, 40, 1361–1403.
Larous, S., & Meniai, A. H. (2016). Adsorption of diclofenac from aqueous solution using activated carbon prepared from olive stones. International Journal of Hydrogen Energy, 41, 10380–10390.
Li, B., & Zhang, T. (2010). Biodegradation and adsorption of antibiotics in the activated sludge process. Environmental Science and Technology, 44, 3468–3473.
Li, G., Ben, W., Ye, H., Zhang, D., & Qiang, Z. (2018). Performance of ozonation and biological activated carbon in eliminating sulfonamides and sulfonamide-resistant bacteria: a pilot-scale study. Chemical Engineering Journal, 341(1), 327–334.
Liu, N., Huang, W., Li, Z., Shao, H., Wu, M., Lei, J., et al. (2018). Radiolytic decomposition of sulfonamide antibiotics: implications to the kinetics, mechanisms and toxicity. Separation and Purification Technology, 202, 259–265.
Liu, M., Zhang, D., Han, J., Liu, C., Ding, Y., Wang, Z., et al. (2020). Adsorption enhanced photocatalytic degradation sulfadiazine antibiotic using porous carbon nitride nanosheets with carbon vacancies. Chemical Engineering Journal, 382, 123017.
Manjunath, S. V., Baghel, R. S., & Kumar, M. (2020). Antagonistic and synergistic analysis of antibiotic adsorption on Prosopis juliflora activated carbon in multicomponent systems. Chemical Engineering Journal, 381, 122713.
Martucci, A., Cremonini, M. A., Blasioli, S., Gigli, L., Gatti, G., Marchese, L., et al. (2013). Adsorption and reaction of sulfachloropyridazine sulfonamide antibiotic on a high silica mordenite: a structural and spectroscopic combined study. Microporous and Mesoporous Materials, 170, 274–286.
Martucci, A., Braschi, I., Marchese, L., & Quartieri, S. (2014). Recent advances in clean-up strategies of waters polluted with sulfonamide antibiotics: a review of sorbents and related properties. Mineralogical Magazine, 78(5), 1115–1140.
Milonjić, S. K. (2007). A consideration of the correct calculation of thermodynamic parameters of adsorption. Journal of the Serbian Chemical Society, 72(12), 1363–1367.
Ncibi, M. C., & Sillanpää, M. (2015). Optimized removal of antibiotic drugs from aqueous solutions using single, double and multi-walled carbon nanotubes. Journal of Hazardous Materials, 298, 102–110.
Peiris, C., Gunatilake, S. R., Mlsna, T. E., Mohan, D., & Vithanage, M. (2017). Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Bioresource Technology, 246, 150–159.
Piccin, J. S., Dotto, G. L., & Pinto, L. A. A. (2011). Adsorption isotherms and thermochemical data of FD&C Red No 40 binding by chitosan. Brazilian Journal of Chemical Engineering, 28(2), 295–304.
Polubesova, T., Zadaka, D., Groisman, L., & Nir, S. (2006). Water remediation by micelle-clay system: case study for tetracycline and sulfonamide antibiotics. Water Research, 40(12), 2369–2374.
Puckowski, A., Mioduszewska, K., Łukaszewicz, P., Borecka, M., Caban, M., Maszkowska, J., et al. (2016). Bioaccumulation and analytics of pharmaceutical residues in the environment: a review. Journal of Pharmaceutical and Biomedical Analysis, 127, 232–255.
Rai, M. K., Giri, B. S., Nath, Y., Bajaj, H., Soni, S., Singh, R. P., et al. (2018). Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from almond shell: kinetics, equilibrium and thermodynamics study. Journal of Water Supply: Research and Technology-AQUA, 67(8), 724–737.
Rezma, S., Birot, M., Hafiane, A., & Deleuze, H. (2017). Physically activated microporous carbon from a new biomass source: date palm petioles. C. R. Chimie, 20, 881–887.
Rizzi, V., D’Agostino, F., Fini, P., Semeraro, P., & Cosma, P. (2017). An interesting environmental friendly cleanup: the excellent potential of olive pomace for disperse blue adsorption/desorption from wastewater. Dyes and Pigments, 140, 480–490.
Sahu, O., & Singh, N. (2019). Significance of bioadsorption process on textile industry wastewater, Chapter 13, Shahid-ul-Islam, B.S. Butola (Ed.), The impact and prospects of green chemistry for textile technology (367–416), Elsevier.
Saka, C. (2012). BET, TG-DTG, FTIR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. Journal of Analytical and Applied Pyrolysis, 95, 21–24.
Sarmah, A. K., Meyer, M. T., & Boxall, A. B. A. (2006). A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere, 65, 725–759.
Shen, G., Zhang, Y., Hu, S., Zhang, H., Yuan, Z., & Zhang, W. (2018). Adsorption and degradation of sulfadiazine and sulfamethoxazole in an agricultural soil system under an anaerobic condition: kinetics and environmental risks. Chemosphere, 194, 266–274.
Singh, K. K., Rastogi, R., & Hasan, S. H. (2005). Removal of Cr (VI) from wastewater using rice bran. Journal of Colloid and Interface Science, 290, 61–68.
Sittig, S. (2014). Sorption, transformation and transport of sulfadiazine in a loess and a sandy soil. Forschungszentrum Jülich: Reihe Energie & Umwelt / Energy & Environment.
Sittig, S., Kasteel, R., Groeneweg, J., Hofmann, D., Thiele, B., Köppchen, S., et al. (2014). Dynamics of transformation of the veterinary antibiotic sulfadiazine in two soils. Chemosphere, 95, 470–477.
Subramani, B. S., Shrihari, S., Manu, B., & Babunarayan, K. S. (2019). Evaluation of pyrolyzed areca husk as a potential adsorbent for the removal of Fe2+ ions from aqueous solutions. Journal of Environmental Management, 246, 345–354.
Sukul, P., Lamshöt, M., Zühlke, S., & Spiteller, M. (2008). Sorption and desorption of sulfadiazine in soil and soil-manure systems. Chemosphere, 73(8), 1344–1350.
Szymanska-Chargot, M., & Zdunek, A. (2013). Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophysics, 8, 29–42.
Tačić, A., Nikolić, V., Nikolić, L., & Savić, I. (2017). Antimicrobial sulfonamide drugs. Advanced Technologies, 6(1), 58–71.
Teixidó, M., Pignatello, J. J., Beltrán, J. L., Granados, M., & Peccia, J. (2011). Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar). Environmental Science and Technology, 45(23), 10020–10027.
Tempkin, M. J., & Pyzhev, V. (1940). Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochimica URSS, 12, 217–222.
Tiwari, B., Sellamuthu, B., Ouarda, Y., Drogui, P., Tyagi, R. D., & Buelna, G. (2017). Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresource Technology, 224, 1–12.
Unold, M., Šimůnek, J., Kasteel, R., Groeneweg, J., & Vereecken, H. (2009). Transport of manure-based applied sulfadiazine and its main transformation products in soil columns. Vadose Zone Journal, 8, 677–689.
Webber, T. N., & Chakravarti, R. K. (1974). Pore and solid diffusion models for fixed bed adsorbers. Journal of American Institute Chemical Engineering, 20, 228–238.
Xia, M., Li, A., Zhu, Z., Zhou, Q., & Ya, W. (2013). Factors influencing antibiotics adsorption onto engineered adsorbents. Journal of Environmental Sciences, 25(7), 1291–1299.
Xiang, Y., Xu, Z., Wei, Y., Zhou, Y., Yang, X., Yang, Y., et al. (2019). Carbon-based materials as adsorbent for antibiotics removal: mechanisms and influencing factors. Journal of Environmental Management, 237, 128–138.
Xu, J., He, Y., Zhang, Y., Guo, C., Li, L., & Wang, Y. (2013). Removal of sulfadiazine from aqueous solution on kaolinite. Frontiers of Environmental Science & Engineering, 7(6), 836–843.
Xu, R., Wu, Z., Zhou, Z., & Meng, F. (2019). Removal of sulfadiazine and tetracycline in membrane bioreactors: linking pathway to microbial community shift. Environmental Technology, 40(2), 134–143.
Xu, X., Meng, L., Dai, Y., Zhang, M., Sun, C., Yang, S., et al. (2020). Bi spheres SPR-coupled Cu2O/Bi2MoO6 with hollow spheres forming Z-scheme Cu2O/Bi/Bi2MoO6 heterostructure for simultaneous photocatalytic decontamination of sulfadiazine and Ni (II). Journal of Hazardous Materials, 381, 120953.
Yakout, S. M., & El-Deen, G. S. (2016). Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arabian Journal of Chemistry, 9, S1155–S1162.
Yang, W., Zheng, F., Xue, X., & Lu, Y. (2011). Investigation into adsorption mechanisms of sulfonamides onto porous adsorbents. Journal of Colloid and Interface Science, 362, 503–509.
Yang, J., Zhou, S., Xiao, A., Li, W., & Ying, G. (2014). Chemical oxidation of sulfadiazine by the Fenton process: kinetics, pathways, toxicity evaluation. Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes, 49, 909–916.
Yang, Y., Ok, Y. S., Kim, K. H., Kwon, E. E., & Tsang, Y. F. (2017). Occurrences and removal of pharmaceuticals and personal care products (PPCps) in drinking water and water/sewage treatment plants: a review. Science of the Total Environment, 596-597, 303–320.
Yang, Y., Zheng, L., Zhang, T., Yu, H., Zhan, Y., Yang, Y., et al. (2019). Adsorption behavior and mechanism of sulfonamides on phosphonic chelating cellulose under different pH effects. Bioresource Technology, 288, 121510.
Yi, Z., Yao, J., Kuang, Y., Chen, H. I., Wang, F., & Yuan, Z. (2015). Removal of Pb (II) by adsorption onto Chinese walnut shell activated carbon. Water Science & Technology, 72(6), 983–989.
Yu, F., Li, Y., Han, S., & Ma, J. (2016). Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere, 153, 365–385.
Yuan, S., Liu, Z., Yin, H., Dang, Z., Wu, P., Zhu, N., et al. (2019). Trace determination of sulfonamide antibiotics and their acetylated metabolites via SPE-LC-MS/MS in wastewater and insights from their occurrence in a municipal wastewater treatment plant. Science of the Total Environment, 653, 815–821.
Zarfl, C., Matthies, M., & Klasmeier, J. (2008). A mechanistical model for the uptake of sulfonamides by bacteria. Chemosphere, 70, 753–760.
Zhang, T., & Li, B. (2011). Occurrence, transformation, and fate of antibiotics in municipal wastewater treatment plants. Critical Reviews in Environmental Science and Technology, 41, 951–998.
Zhang, L., Wang, Y., Jin, S. W., Lu, Q. Z., & Ji, J. (2017). Adsorption isotherm, kinetic and mechanism of expanded graphite for sulfadiazine antibiotics removal from aqueous solutions. Environmental Technology, 38(20), 2629–2638.
Zhao, H., Xu, J., Liu, X., Cao, Z., Zhan, Y., Shi, X., et al. (2016). Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. Journal of Hazardous Materials, 310, 235–245.
Zheng, H., Wang, Z., Zhao, J., Herbert, S., & Xing, B. (2013). Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environmental Pollution, 181, 60–67.
Acknowledgments
This paper includes a part of the master thesis data of Meltem Gözegir.
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Firat University (FUBAP) (Project Number MF.18.48).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslan, S., Şirazi, M. Adsorption of Sulfonamide Antibiotic onto Activated Carbon Prepared from an Agro-industrial By-Product as Low-Cost Adsorbent: Equilibrium, Thermodynamic, and Kinetic Studies. Water Air Soil Pollut 231, 222 (2020). https://doi.org/10.1007/s11270-020-04576-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04576-0